Environmental monitoring helps VisionGift provide restored sight & renewed life
VisionGift® is a non-profit organization dedicated to the mission of Honoring Donors by Advancing Sight for all Humankind, through eye donation, transplantation, and research. Founded in 1975, VisionGift has facilitated thousands of corneal transplants and supported advances in ocular research and therapy. VisionGift holds accreditation with the Eye Bank Association of America (EBAA), is registered with the U.S. Food and Drug Administration (FDA), and is licensed in several states, including Oregon, California, and Maryland, as well as Canada.
Every year, VisionGift contributes to sight restoration through corneal transplants, including tectonic graft surgery to treat glaucoma, keratoconus and other ocular diseases, and research initiatives for diseases of the eye. Today, cornea transplant surgery has a success rate that exceeds 95%.
A Compliant Point of View
Kristen Mathes has been passionate about tissue banking for over twenty years. As the VP of Quality Systems, Mathes is trained in ISO 13485 Quality Systems, 510(K) clearance processes, and De Novo requirements. She has brought her expertise in quality systems, processing, and regulatory requirements for human cell and tissue products to oversee the regulatory approval of important new products, helping to increase the availability of corneal transplantation and other sight restoration treatments.
“At VisionGift I oversee the Quality Assurance and Regulatory Affairs department, as well as Information Systems,” said Mathes. “We maintain accreditation with the Eye Bank Association of America (EBAA), which is our national accrediting agency. But many states require that we register or get state-specific certification. New York for instance has some unique requirements. Because we place tissue in the European Union and in the UK, we also have to comply with their regulations.”
Timely Tissue Evaluation
When ocular tissue is donated, it goes to the laboratory to be evaluated for either short- or long-term storage. The length of time tissue will be stored largely depends on how it will be used, which is determined by each tissue’s characteristics. Short-term storage tissue, referred as ‘fresh’ tissue, is not irradiated and has living cells necessary for 99% of surgeries that use this type of tissue. It is critical that healthy endothelial cells are used for those transplants. Whether tissue is designated as short- or long-term storage is decided in large part by its endothelial integrity. Even when tissues are not ideal, they can often be considered for long-term storage. These grafts are irradiated and have a longer shelf life.
“The clock starts ticking as soon as the tissue has been recovered. Ophthalmic surgeons are extremely particular about the length of time between date-of-death and date-of-surgery for transplants using fresh tissue. They will typically only use tissue with no more than several days in media. This means that we need to move quickly, especially if the tissue will go to the UK or EU. But, if the donation is acceptable for long-term use, we can store it for up to a year in -80°C freezers before it must be irradiated, giving us a larger window to honor the gifts from a donor and ensure we are being good stewards of their gift.”
Tissue designated for long-term storage is sterilized by E-Beam irradiation and is categorized as human cells, tissues, and cellular and tissue-based products (HCT/Ps). For long-term storage, frozen corneal tissue is kept in media containing recombinant human serum albumin. After irradiation, the tissue can then be safely stored at room temperature for up to two years.
“Long-term tissue, including irradiated cornea, sclera, and pericardium, is used for most of the tissue grafts that we distribute," said Mathes. "These tissues, called halo® sterile tissues, can be used in patch grafts or to protect glaucoma shunts.” Glaucoma shunts are devices that reduce pressure on the eye by draining excess fluid to a small blister behind the eyelid. A patch made of donated eye tissue, either from sclera (white part of the eye) or cornea (clear part of the eye) protects and holds a small tube in place to drain fluid and relieve pressure. For these products, living cells are not needed. These tissues can be processed, frozen and then irradiated for later use.
Advances in Allograft Technology
There are several types of corneal transplants, or keratoplasties. Three of the most common types of corneal transplants are endothelial keratoplasty (which replaces the innermost cell layer of the cornea), penetrating keratoplasty (for complete or full-thickness corneal transplant), and anterior lamellar keratoplasty (for partial corneal transplant on the most anterior part of the cornea).
“One disorder that can be improved or entirely alleviated by corneal transplant is Keratoconus, where the cornea thins and bulges into an irregular cone shape. It can be quite painful, and left untreated, gets progressively worse resulting in blindness,” said Mathes. “Penetrating keratoplasty, using fresh tissue, is one way to treat this disorder. We have developed an irradiated allograft product called KeraNatural® and have been working with Dr. Aylin Kilic and her colleagues at Swiss Vision Group in Istanbul, Turkey to understand the efficacy of these products in treating keratoconus.
“In VisionGift laboratories, donated cornea tissue is cut into an appropriate shape to form a graft and then sent for irradiation. Once in the hands of the surgeon, the graft is inserted into a laser cut tunnel in the cornea of the patient. The graft alters the shape of the cornea to alleviate the coned or bulging shape. They may also use laser surgery to finetune the surface of the eye with the graft. It’s truly some of the most exciting eye surgery in the Ophthalmologic world right now and there are some fantastic results published in an article this year in Nature.*”
viewLinc at VisionGift
From two facilities in Boston MA and Portland OR, VisionGift works hard to honor donors and their families through their "Donor Family Community Group” and “Remembering Heart” programs. VisionGift also facilitates correspondence between donor families and grateful tissue recipients. These projects provide comfort to families and a way for recipients to give thanks.
"We need continuous records of our storage and processing conditions, not only to prove to regulators that the tissues are safe, but to honor the donor and their gift. These tissue products are truly irreplaceable."
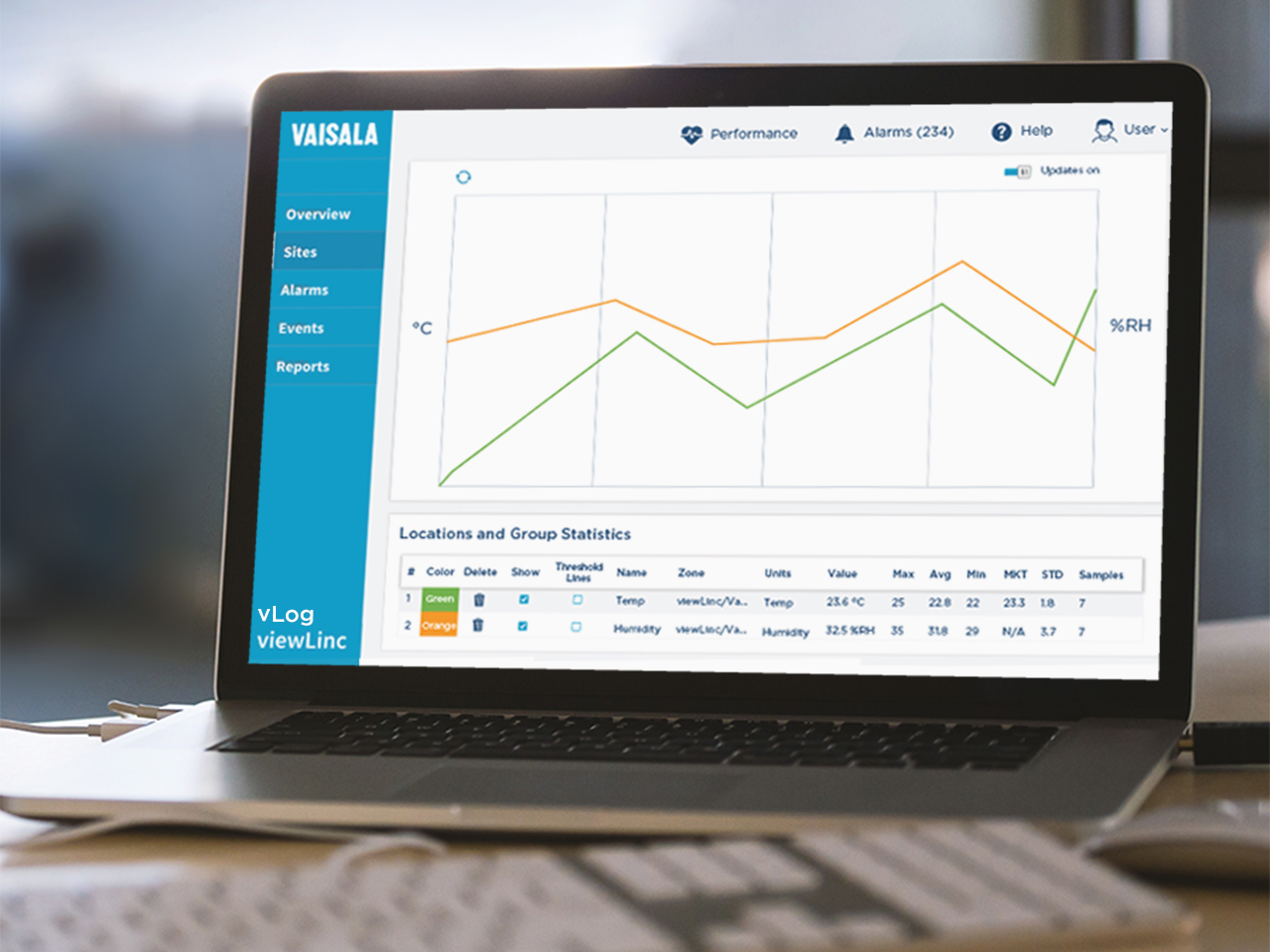
VisionGift has used the viewLinc Continuous Monitoring System since 2011. Previously, most records of environmental conditions were generated by chart recorders and manual records.
“We use the viewLinc system for monitoring storage areas to ensure proper conditions for reagents, supplies, and tissue. We also use the system to monitor our processing areas. We have both ISO 5 and ISO 7 clean rooms,” said Mathes. “The reports we get from viewLinc are ideal for compliance. Inspectors will go through them line by line, ensuring all the thresholds are properly set and maintained,” said Mathes.
The viewLinc system can send remote and local alerts of out-of-tolerance conditions by email, SMS, voice call, lights, and buzzers. Alarms can be acknowledged on mobile phones via voice call, SMS, or email. “One of the advantages with viewLinc is that we can configure alarms to notify the relevant staff if the conditions are out of tolerance,” said Mathes. “It’s convenient that viewLinc allows us to schedule notifications to the right personnel, at the right location, at times we know they’ll be able to respond.”
A Different Kind of Bank
In 2021, VisionGift experienced a break-in. “I’m not sure what they thought they were going to steal… it’s conceivable they actually thought we were a financial bank rather than a tissue bank!” said Mathes. “But the thieves cut off the power, which knocked out both the HVAC system and network. Although all they took was a couple of laptops, it took many hours to get things back online.
“At that time, our viewLinc system was on an on-premise server. We’ve since migrated viewLinc to a cloud server,” said Mathes. “Once the electricity was back on, we started getting alarms from viewLinc. To mitigate any damage as we waited for conditions to return to normal, we kept all storage chambers closed. What we didn’t know, at that time, was that when the thieves cut the power, it caused a compressor failure in one of the freezers…”
As power was restored Mathes remotely logged into viewLinc and watched as system’s sensors back-filled environmental data to the server. She noticed that one of the freezers was not returning to its correct temperature. “It was late at night, and no one was in the office, so I drove to the eye bank and moved all of the tissue in that freezer into another, fully functional freezer,” recalls Mathes.
“I printed the historical reports from viewLinc and sent them to an infection disease expert. We needed to know, based on the temperature records, if the tissue was still safe. Thankfully, it was. It was critical that the sensors had on-board power and data storage, so even with the power cut off, we had records of the conditions.”
Tissue transplantation is one of the most important medical advances of modernity. With over 2.5 million procedures performed each year for a variety of injuries and diseases, tissue transplants can allow a single donor to save and heal the lives of more than seventy-five other people.