Reliable data. Where to place sensors & why.
Temperature and Humidity Validation Mapping

Mapping Made Easy: Where to Place Sensors & Why
Topics
- A review on global regulatory changes and guidance on GDP
- Techniques that ensure storage spaces meet specifications
- Review crucial factors that impact sensor placement
- Tips on creating an effective ratio of sensor to area volume for mapping

5 Rules of sensor placement in validation/mapping applications
This article expands upon 5 rules to apply when creating a rationale for sensor placement in mapping studies. Learn how to:
- Map the extremes
- Map in three dimensions
- Map large spaces
- Identify and address variables
- Relationship of mapping and monitoring

5 Frequently Asked Questions about Temperature and Humidity Validation Mapping
- What limits should I use as an acceptable range for my study?
- What type of sensor(s) should I use?
- How many sensors do I need, and where should I place them?
- What kind of calibration do my sensors require?
- What is the appropriate duration for a mapping study?
Validation Mapping

Temperature and Humidity Data Loggers DL2000

DL1000-1400 Temperature Logger

Temperature Data Loggers DL1016/1416
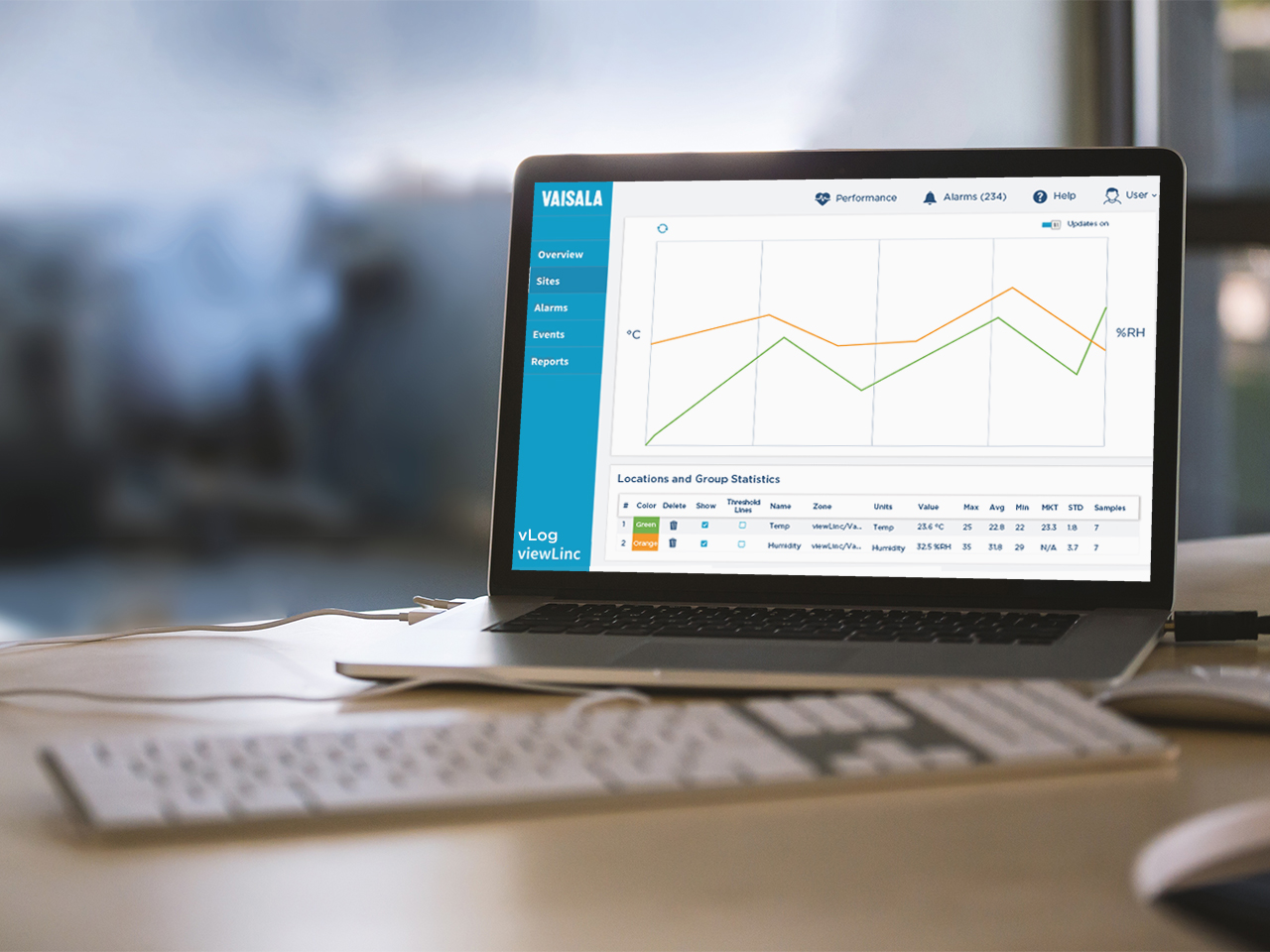
vLog VL software
IQOQ Sample
The purpose of this IQ/OQ protocol is to provide assurance that the Vaisala vLog Data Logging and Reporting System has been set up properly, is functional, and operates with a high degree of integrity, security and reliability. The Installation Qualification (IQ) protocol template has been designed to ensure that the system, composed of both the hardware and the software, has been installed correctly at the point of use. The Operation Qualification (OQ) protocol template has been designed to ensure that each component of the system performs as intended.

Contact our Validation Mapping Team
Get all your questions answered!

Life Science eBook
Vaisala solutions help you safeguard sensitive products in warehouses, processing/manufacturing facilities, laboratories and clean rooms. Download our brand new Life science eBook today and start making informative decisions on preserving the integrity of your products.