STERIS provides efficient, effective bio-decontamination with accurate VHP sensors
STERIS provides a wide range of vaporized hydrogen peroxide (VHP®) bio-decontamination systems and services, utilizing Vaprox® Sterilant for broad spectrum efficacy against viruses, bacteria, yeasts, and bacterial spores. Vaporized hydrogen peroxide bio-decontamination is crucial, not only for pharmaceutical and biotechnology production, but also for agricultural industries and healthcare facilities. The STERIS bio-decontamination systems use a “dry process” hydrogen peroxide vapor distribution, which eliminates the risk of condensation on surfaces. The advantages of decontamination with vaporized H2O2 include:
• Easy to use
• Effective against biological contaminants
• Ideal for low-temperature processes
• Processes can be validated
• Compatible with a wide variety of materials
• Environmentally friendly and safe for operators
• Leaves no toxic residue, only water vapor and oxygen
A trusted partner
For several years, STERIS has used a trusted sensing technology from Vaisala. In 2018, STERIS became interested in a new solution from Vaisala: the HPP270-series hydrogen peroxide vapor probe. The probes feature PEROXCAP® sensing technology. The sensors provide accurate measurements for hydrogen peroxide concentration or ppm (parts per million) and several other parameters, most importantly: relative humidity, temperature, and a new parameter: relative saturation — which indicates when condensation will occur.
Validating VHP bio-decontamination
In DSVA (surface disinfection by airways) the aim is to prove that bacteria and microorganisms have been eradicated and the results must demonstrate maximum effectiveness throughout the bio-decontaminated area. To validate a cleanroom bio-decontamination, it is essential that STERIS use a highly accurate sensor that can provide stable, repeatable data on the concentration of hydrogen peroxide vapor ppm. Vaisala’s unique technology met STERIS’s requirements for measurement reliability and repeatability. Thanks to Vaisala, STERIS has been able to prove maximum effectiveness of bio-decontamination in clean rooms.
“Today, Vaisala is the best technology on the market to measure the concentration of H2O2 reliably, accurately, and repeatably over time,” says Philippe Muylaert, Room Decontamination Service Specialist with STERIS SAS. “The Vaisala model HPP272 is the most effective sensor for bio-decontamination with hydrogen peroxide. Thus, we have confidence in the data, and we can prove to our customers the effectiveness of bio-decontamination cycles.”
Muylaert appreciates that the HPP270 probes provide H2O2 concentration measurement curves throughout the bio-decontamination process in addition to real-time monitoring data. In-line data throughout a process is valuable for cycle development, especially to help determine pressure binary mixture, water concentration, temperature, etc.
In-line measurement of VHP
To remain in a gaseous state, hydrogen peroxide vapor requires controlled parameters, including temperature, relative humidity, pressure and volume. Any departure from ideal conditions can cause hydrogen peroxide vapor to condense, essentially returning the H2O2 to its natural state: liquid.
For STERIS’s dry method, it is necessary to avoid condensation that can lead to equipment deterioration. In the absence of hydrogen peroxide vapor, the relative humidity of the air is equal to the relative saturation (1). When vaporized H2O2 is introduced, the relative saturation is greater than the relative humidity (2).
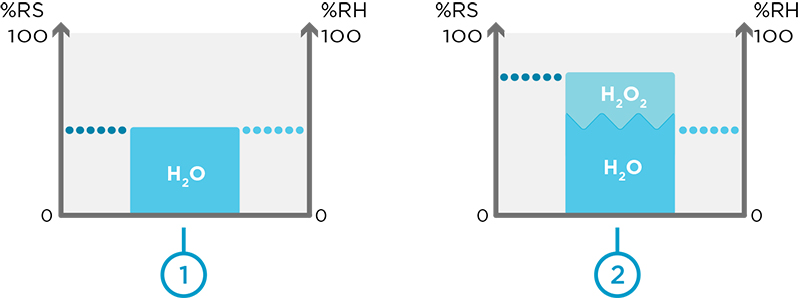
During H2O2 vapor bio-decontamination processes, there is always water vapor in addition to hydrogen peroxide vapor. To control condensation, you need to know both the humidity of the air caused by water vapor and by hydrogen peroxide vapor. Relative saturation, which indicates the concentration of vaporized hydrogen peroxide and water vapor in the air, is the only value that represents both vapors. Monitoring the relative saturation value during a process is therefore crucial, because it indicates saturation point of the combined vapors: water and hydrogen peroxide.
Reliable measurements mean reliable processes
STERIS systems required a probe capable of providing accurate measurements for hydrogen peroxide ppm, temperature, relative humidity and relative saturation. Using the unique Vaisala PEROXCAP® hydrogen peroxide sensor technology, the HPP272 probe can also measure two other parameters: dew point and vapor pressure, which can also be useful parameters in bio-decontamination.
The probe guarantees reliable and precise hydrogen peroxide measurements throughout the bio-decontamination cycle, even in high humidity. The reliable and reproducible measurements of Vaisala’s vaporized hydrogen peroxide probes allow STERIS to achieve a high degree of confidence in their bio-decontamination procedures, success during annual audits, and a high level of product quality.


