Science-based skincare benefits from continuous monitoring & efficient validation
Established in 2000, Crown Laboratories is a global company that researches, develops, and manufactures skincare products ranging from over-the-counter topical therapies to medical devices and prescription formulations. Crown Laboratories is involved in planning clinical trials that are advancing the science of dermatology. Their products fall into four main categories: Aesthetics, Premium Skincare, Therapeutic, and Prescription.
Environmental monitoring data for regulatory compliance
Even for lower risk categories like cosmetics, the manufacturing and distribution of cosmetics differs around the world and each nation oversees regulation individually. In the European Union, the EU Cosmetics Directive requires that manufacturers maintain complete technical dossiers of products, which are reviewed by local authorities. In the United States, cosmetics are regulated by the U.S. Food and Drug Administration (FDA), which has regulatory authority under the Food, Drug and Cosmetic Act, specifically FDA Title 21 Chapter I Subchapter G Cosmetics. The FDA conducts inspections and collects samples to verify product safety.
In Japan, cosmetics are regulated through the Ministry of Health, Labor, and Welfare according to the Pharmaceutical Affairs Law (Law No. 145). In Canada, the national agency, Health Canada, has a Cosmetics Program that includes products used by aesthetics practitioners, as well as bulk products. In effect, each country seeks to safeguard the end user through regulatory oversight. To meet these requirements, manufacturers ensure they collect data on the conditions in which prescription and over-the-counter drugs, medical devices, and cosmetics are manufactured, transported, and stored.
In recent years, many cosmetics manufacturers have increased their investments in facilities, technology, and human resources to ensure that products are safe, effective, and compliant with regulations. In addition, some companies also conduct scientific research and employ chemists, toxicologists, microbiologists, and other experts. These investments not only ensure quality and safety, but they also allow for innovation for science-backed cosmetics and skin therapeutics.
Good Manufacturing Practice Provides State-of-the-art Skincare
Mary Gilbert is the Senior Quality Engineer at Crown Laboratories. She is experienced in Validation and Equipment Qualification and is an expert at creating test plans, writing, and executing validation protocols, and assessing or reviewing Validated or Qualified systems and equipment under Change Control.
In her Crown Laboratories role, she manages Critical Utility and Controlled Systems projects and management of change. This includes system configuration, applicable Computer Systems Validation, Quality Monitoring and Mapping of Environmental Conditions and system equipment as well as document reviews and approvals.
“Our portfolio offers skincare solutions throughout our lifetime skincare journey, from infancy to older adults. A good example is the “Blue Lizard® line of sunscreen products. We have a baby formulation, a kids’ version, and a sport sunscreen that is water repellant,” says Gilbert. “But the products are wide-ranging; for instance, we have a micro-needling device, SkinPen® Precision.” This product was the first FDA-cleared device clinically proven to improve neck wrinkles and facial acne scars.”
Crown Laboratories maintains cGMP-compliant manufacturing operations to provide services that include research and development, lab-scale formulation, manufacturing and packaging, analytical labs, method development and validation, testing for uniformity, hold-time, and ICH stability.
“We are audited by the FDA and firms for whom we manufacture,” says Gilbert. “No matter who is conducting the audit, they want to see data from our monitoring system and sometimes the entire validation package data. It depends on the application. For instance, as a medical device, SkinPen® Precision has ICH humidity requirements and is audited by a 3rd party for ISO 13485 certification. Because our products are regulated, our operations must adhere to Current Good Manufacturing Practice as well as to applicable certifications.”
Environmental Monitoring Ensures Quality
Crown Laboratories uses the Vaisala viewLinc Continuous Monitoring System to ensure its controlled areas operate under proper environmental conditions. “We started with the viewLinc monitoring system in stability chambers,” says Gilbert. “Later we expanded viewLinc to monitor warehouses and laboratories.”
In 2018, Crown added the VaiNet wireless data loggers to their viewLinc monitoring system. VaiNet technology operates independently of Wi-Fi and other wireless devices, reducing the load on other networks. VaiNet AP10 network access points can support 32 wireless data loggers, eliminating the need for dedicated Ethernet connectivity for each monitored location.
“The system is easy to set up,” says Gilbert. “Once you’ve configured the AP10 networking device, it connects automatically with RFL100 data loggers. For example, yesterday I placed a probe from an RFL100 into a freezer. You simply get the AP10 to detect the data logger and the AP10 sends the data to viewLinc.”
Early Detection Saves Costs
The viewLinc monitoring system sends remote and local alerts of out-of-specification conditions to designated personnel. Alerts can be sent via email, SMS, voice call, lights, and buzzers.
“Recently we had an event on a stability chamber where viewLinc helped us determine a problem before it became destructive,” says Gilbert. “Whenever a new product is developed it needs stability data to support its approval. For instance, if you use a different tank or filler, or you need to change an ingredient, anything that might affect its efficacy, it requires stability testing.
“In this particular chamber, the trend data in viewLinc showed decreasing humidity. As soon as I saw the trend, I contacted our maintenance lead. He checked the chamber and determined that the heater on the humidifier had failed. We quickly relocated the product to another chamber until maintenance could make the repair.”
“I was happy to alert the Stability team so they could identify a root cause before the chamber went into alarm. The real-time data in viewLinc keeps you on top of what’s going on in your facility. This data enabled us to switch to a backup chamber before the study was impacted and gave us time to schedule repairs.”
Mary Gilbert, Senior Quality Engineer, Crown Laboratories
Months of data can be retained in the memory of each viewLinc data logger. Automatic data backfill to viewLinc’s server ensures gap-free data during network or power outages.
“We sometimes experience brief power outages,” says Gilbert. “Although we have all critical equipment running on a backup generator, it’s good that the AP10s will send a communication alert that it has lost connection. Once power is back, the AP10 automatically re-connects and backfills the data to viewLinc from the battery-powered data loggers.”
Flexible Parameters
The viewLinc system can integrate unlimited parameters with Modbus TCP/RTU and analog devices.
“In 2021, I contacted Vaisala to see if devices with a 4-20 mA signal could send on/off readings to viewLinc,” says Gilbert. “Our engineering team needed a way to study compressed air usage. We used Vaisala’s DL4000 universal input data loggers to gather data on two flow meters. This information was provided to an engineering firm for the design of an upgraded compressed air system. This project also used a Vaisala DMT152 dew point sensor for monitoring and alarming of desiccant compressed air.”
Mapping with viewLinc
Although viewLinc is a monitoring system, mapping studies can be performed with the software and data loggers. In both monitoring and mapping applications, environmental data is collected at regular intervals. The primary difference is that in monitoring, the data loggers are permanently deployed in a location and the data can be viewed live. In a mapping study, the data loggers are typically deployed for shorter periods in locations that change from study to study.
“In previous mapping studies we used Vaisala DL-series data loggers with Vaisala’s vLog software,” says Gilbert. “In 2021, our IT team transitioned our computers to a new operating system. As part of continuous improvement and to ensure robust backup and data security, Curtis Unger at Vaisala’s Helpdesk suggested we replace vLog which was validated on an older operating system to viewLinc for mapping studies.
“Using viewLinc for mapping has some real benefits. First, we now have only one software to validate. Second, I am more familiar with viewLinc’s interface because I use it daily, whereas I used vLog three times a year for validation. When I used vLog, I would run five or six reports, three in vLog with low, central, and high-end values and two or three in viewLinc to capture my monitoring loggers. For warehouse mapping, I’d have sensors placed low, central, and high for mapping and monitoring. To get the same data using viewLinc, I simply had to add my mapping loggers to the software. Now I only print three reports. It’s convenient that I can keep the loggers where I want them, inactivate them when I’m not mapping, then activate them for study purposes.”
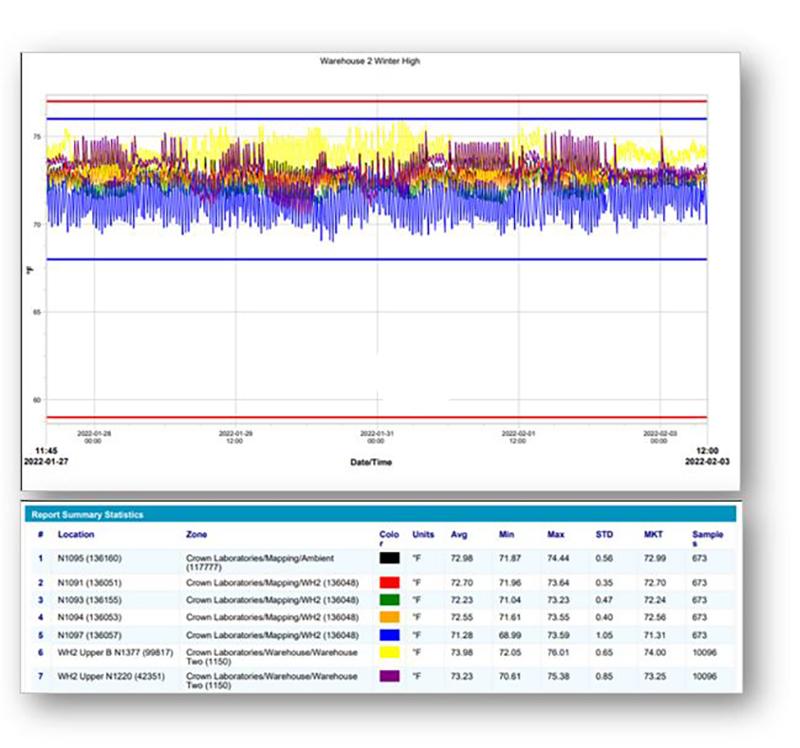
When using viewLinc to download data from mapping loggers, viewLinc’s backfill process is an advantage. Once data loggers are connected to viewLinc after a mapping study, the software regards data loggers as temporarily disconnected and automatically begins downloading data stored in the logger’s local memory. Using viewLinc for mapping is an efficient alternative to thermocouple-based equipment, data acquisition systems, and less robust data loggers. The software can be used to map any typical GxP storage environment, including stability chambers, refrigerators, freezers, incubators, warehouses, ambient environments, and other environments.
“I use the Site Manager in the viewLinc software to keep monitoring and mapping data separate,” says Gilbert. “This keeps the interface clean and focused on monitoring. I simply disabled the configuration and communication alarms for the mapping loggers to avoid nuisance alarms when we are not performing mapping studies.”
“The data provided by viewLinc allows us to make a data-driven decisions.”
Mary Gilbert, Senior Quality Engineer, Crown Laboratories
Application note: Temperature Mapping with the viewLinc Monitoring System
