Built to survive, even outside: the Vaisala Indigo500 Series

Some of our customers have applications that require measurement transmitters to be installed outside, so the Vaisala Indigo500 and its family of smart probes have been designed to be durable enough for these kinds of rough, tough conditions. For example, when measuring humidity in gas turbine intake air or in combustion air for diesel generator stations you need the measurement devices to be outdoors. There are also applications where it’s not desired, but outdoor measurement will be dictated by practical considerations.
In the majority of outdoor applications, the most important parameters to measure are usually humidity and temperature, but moisture-in-oil measurement for transformer applications is also necessarily an outdoor application. Another common outdoor application is CO2 measurement for demand control ventilation in buildings – this sort of application really benefits from the Indigo500’s ability to support multiparameter configurations as you can keep the instrument on the roof of your building and use another probe to measure relative humidity, for example.
The three major challenges of outdoor use
Instruments for outdoor use need to be robust enough to stand up to three main challenges: a wide temperature range, the weather, and solar radiation or UV. Temperature is one of the easiest parameters to validate, and the Indigo500 has been tested and validated in a climate chamber to ensure that the device can not only withstand the extremes of outdoor temperatures, they can also endure the rapid changes of dynamic, cycling temperatures.
The main challenge of weather is water ingress into the device, so we validated the Indigo500 using Ingress Protection (IP) testing, which involves exposing the unit to water sprays, ensuring the robustness of the overall mechanical construction. Another way in which water can enter the housing is as a vapor, and this has also been taken into account in the design. A breathing element on the reverse allows water vapor to stabilize between the inside and outside of the transmitter housing, preventing condensation and pressure changes inside the housing arising from the changing temperature. The latter may cause under pressure in the housing when the warm enclosure is exposed to cool water showers, which in turn increases the possibility of water ingress into the housing – a test called “tropical rain” by test engineers.
To protect against solar radiation, we chose aluminum alloy as a construction material because it withstands UV and won’t degrade. A special surface treatment provides protection from oxidation and rust build up. All these factors have been designed into the Indigo500 and validated in environmental tests throughout the R&D process to make sure it can stand up to the demands of all kinds of outdoor applications. Finally, we’ve been testing the Indigo500 outside in the field for the last two winters in southern Finland, with no problems to report despite constant damp and rainy weather.
Accurate measurements inside or out
Connecting the Indigo500 to your current systems and operations is the same process whether your application is indoors or out. Accurate measurement data can be transferred using the same system interfaces and connectivity, including analog outputs, binary contact relays, Ethernet, and local interfaces such as the touch panel display.
Although outdoor applications are not the primary use for the Indigo500, we followed strict design requirements to ensure it meets the same standards as outdoor devices. And of course, if it is tough enough for outdoor applications, it means it is also robust and dependable for indoor applications too. Wherever you use it, the Indigo500 is designed to be relied upon for dependable and accurate measurement data for many years.
Learn more about Indigo500 Series Transmitters >
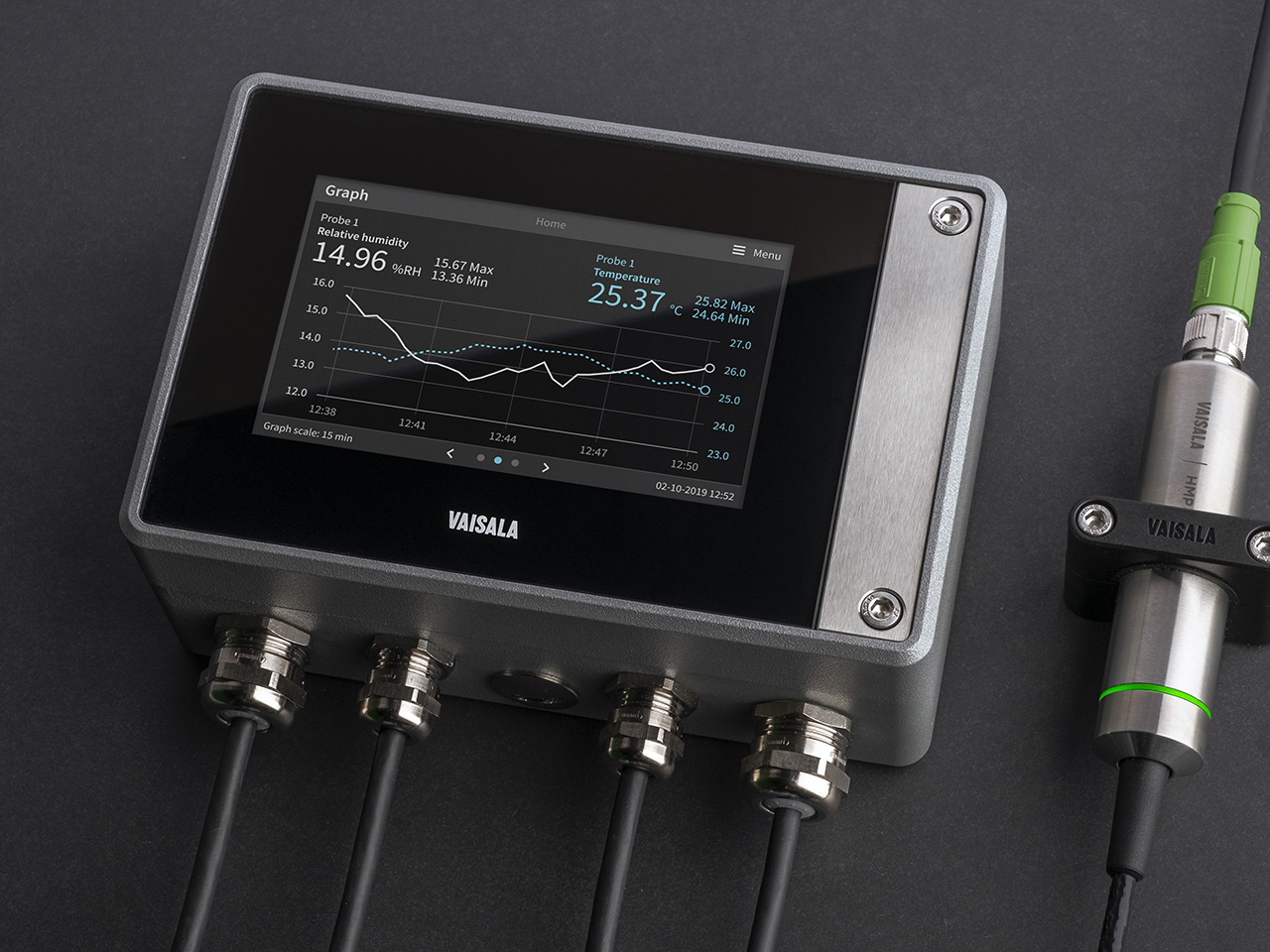
Indigo500 Series Transmitters
The Vaisala Indigo500 series transmitters are host devices for Vaisala Indigo-compatible, stand-alone smart probes. The Indigo500 series include multi-functional Indigo520 transmitter and Indigo510 transmitter with basic features.

Humidity and Temperature Probe HMP3
Vaisala HUMICAP® Humidity and Temperature Probe HMP3 is a general-purpose probe designed for processes with moderate humidity and temperature levels.

Relative Humidity and Temperature Probe HMP7
Vaisala HUMICAP® Humidity and Temperature Probe HMP7 is designed for applications which involve constant high humidity or rapid changes in humidity

Temperature Probe TMP1
Vaisala Temperature Probe TMP1 is designed for demanding temperature measurements in industrial applications such as pharmaceutical industry and calibration laboratories, where accuracy and robustness are essential.

CO₂ Probe GMP252
The Vaisala CARBOCAP® Carbon Dioxide Probe GMP252 is an intelligent, stand-alone, ppm-level probe. It's intended for measuring CO2 in agriculture, refrigeration, greenhouses, demanding HVAC applications, and for plant growth chamber manufacturers.

















Thanks.