Fighting superbugs proactively: innovation and collaboration in vaporized hydrogen peroxide bio-decontamination

In 2014, an independent report commissioned by the UK estimated that drug-resistant infections could result in 10 million deaths and cost over 100 trillion USD by 2050. (See: “Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations.”) Drug-resistant infections, or so-called “superbugs,” include Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant Enterococcus (VRE), Clostridium difficile (C. difficile), Candia Auris, and other resistant organisms. In response to this emerging issue, the Secretary-General of the United Nations created The Interagency Coordination Group (IACG) on Antimicrobial Resistance in 2016. The IACG supplied its report to the UN in April of 2019, “No time to Wait: Securing the future from Drug-resistant Infections.”
Innovate & collaborate
The report makes five recommendations to combat the threat of Antimicrobial Resistance (AMR), including: “Innovate to Secure the Future” and “Collaborate for more Effective Action.” In Finland, collaboration and innovation to combat drug-resistant pathogens are occurring between the VTT Technical Research Centre of Finland Ltd, Cleamix, a manufacturer of portable hydrogen peroxide vapor generators, and industrial measurement system and sensor manufacturer Vaisala Oyj.
This particular story of innovation begins with the Finnish Air Force seeking a way to destroy biological toxins and weaponized microorganisms. The US military had done a lot of initial work showing that vaporized hydrogen peroxide could be effective as a bio-decontaminant. The problem was that most commercially available H2O2 vapor generators were too large to be field-deployed. So, the Finnish military turned to the scientific community to find a vapor generator that was portable, cost-effective, and capable of sufficient hydrogen peroxide vapor output.
Finnish equipment manufacturer Cleamix was founded to study the problem and created a lightweight vapor generator that could produce sufficient quantities of vapor with the required concentrations of hydrogen peroxide. However, to ensure the vapor would be effective in destroying microorganisms, Cleamix needed their device to specify the right concentration of H2O2 vapor over a given time period. That required a sensor that could measure both the concentration of hydrogen peroxide vapor, as well as other critical process parameters including temperature and a humidity value derived from the combination of water and hydrogen peroxide vapor: Relative Saturation RS%.
“Whether you are decontaminating a cockpit, ambulance, isolator or operating room (really any area that can be contaminated), you need inline sensors that give values not only for the H2O2 vapor but also for the Relative Saturation, because this will tell you when condensation will occur at the current temperature. Relative saturation indicates the humidity value derived from the combination of water and hydrogen peroxide vapor.” - Panu Wilska, CEO Cleamix
Private enterprise working for public interests
Panu Wilska came to Cleamix in 2016 with over 25 years of international experience ranging from nuclear physics to managing hi-tech start-ups. He has served the company as an advisor, board member, board chair, and now CEO. Cleamix learned that Vaisala was developing a sensor for vaporized hydrogen peroxide and that the sensor would give multiple values; ppm of H2O2 and temperature, but most importantly, a value for the saturation point. Although it is technically possible to calculate values for each parameter - temperature, relative humidity, and ppm H2O2 - you still need a sensor for each.
New technologies combined
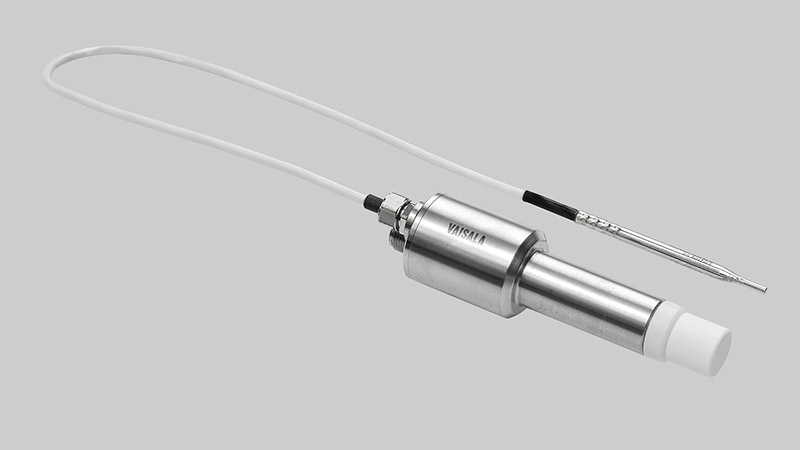
Vaisala created the PEROXCAP® technology, and Cleamix was one of the companies that tested the first probes in the series (HPP270). The probes can be used with vapor generators to measure conditions under decontamination; the probes can also be integrated to control the vapor output according to process requirements. Because the Vaisala HPP-series probes enable real-time process control, if the Cleamix vapor generator needs to adjust output to match changing environmental conditions during a process, the probe data allows the generator to adjust automatically.
Cleamix also worked with the military on subsequent testing that was performed at a military research center using vaporized hydrogen peroxide as a biocide in abandoned military buildings to identify required concentration levels of vaporized hydrogen peroxide. Cleamix initially created two models of portable generators. The larger model weighs only 9.5 kg and can decontaminate areas from ten cubic meters and upwards. Several vapor generators can be networked for large areas, typically using one vaporizer per 100 cubic meters. The smaller model weighs 6 kg and is ideal for areas one to 20 cubic meters, including: cabinets and enclosures, laboratory cabinets, and vehicles, like ambulances and aircraft. Independent tests with the Cleamix units have shown the vapor efficiency ratio (the amount of aqueous hydrogen peroxide that vaporizes) exceeds 90%.
Efficient, effective bio-decontamination
The Cleamix generator uses about one liter of liquid H2O2 for five and a half hours of continuous, full-power operation. With a combination of phase change methods, accelerated vaporization under and over atmospheric pressure, and 3rd party validated efficacy; the company now has 26 patents pending for its unique technology.
Other innovations followed. Along with its portability, the Cleamix vapor generators can vaporize a combination of liquids. Typical applications require a 50% H2O2 aqueous solution, but with the addition of a small amount of ammonia, the vapor can destroy other pathogens, including weaponized nerve gas. The Cleamix H2O2 vapor generators have been independently tested by two different military organizations and found to successfully neutralize all nerve agents, including VX and Sarin. This year saw testing of the Cleamix units in laboratories researching a dangerous new superbug, Candida Auris (C.Auris).
This rapidly emerging fungal pathogen was first discovered in Japan in 2009 and can cause life-threatening infections due to its resistance to all three classes of antifungals. Cleamix’s tests have shown that acetic acid will accelerate decontamination, but C. Auris can also be destroyed with high-concentration vaporized hydrogen peroxide alone.
Emerging threat meets state-of-the-art solution
In April of 2019, the New York Times published an article on C. Auris titled: “A Mysterious Infection, Spanning the Globe in a Climate of Secrecy.” The article describes recent outbreaks in hospitals and medical centers in Spain, the UK, and several states in the US. The Centers for Disease Control and Prevention have added C.Auris to its list of urgent threats. Globally, C.Auris outbreaks have occurred in India, Pakistan, and South Africa. Both the Public Health Agency of Canada (PHAC) and the South African Centre for Opportunistic, Tropical and Hospital Infections (COTHI), released interim recommendations on the management of C. Auris suggesting the use of hydrogen peroxide vapor when feasible, in addition to other decontamination agents and methods. (See: “Candida Auris: Disinfectants and Implications for Infection Control.”)
“C.Auris is highly resistant to many biocides, including vaporized H2O2, but it can effectively be destroyed by H2O2 vapor mixed with other agents. The other liquid used must be more acidic, like Peracetic or Acetic acid. We are involved in more testing with various labs. This is why it was critical that the vaporization method of Cleamix units allowed for combination of liquids.
“Bio-decontamination with vaporized hydrogen peroxide can be used proactively, not reactively. These pathogens are hard to kill and even harder to cure once a person is infected. Frequent bio-decontamination can stop outbreaks, but the equipment needs to be portable, highly efficient, and affordable.” - Panu Wilska, Cleamix
The birth of a parameter
By the time Cleamix began to work with Vaisala, they had already tested other hydrogen peroxide sensors but needed a sensor that was stable, accurate, easy to integrate, and able to provide measurements for all the necessary parameters. “We needed a device that could give a value for the relative saturation of the mixture of water vapor and H2O2 vapor because our original tests used a “dry method” of bio-decontamination that avoided visible condensation,” says Wilska.
Vaisala engineers created a sensor that could measure and control the most important parameters during bio-decontamination: ppm H2O2, humidity, and temperature. This gave rise to a new parameter: Relative Saturation. This parameter helps operators ensure that a process either avoids condensation (dry method vapor decontamination), or includes condensation (wet process). Equipped with Vaisala’s new PEROXCAP® technology in the HPP270 series probes, Cleamix units provide known H2O2 concentration values. The key process parameters in bio-decontamination are H2O2 ppm concentration, temperature, relative humidity, and exposure time.
In pharmaceutical research, development, and production, bio-decontamination between batches or processes is critical to product quality. In many cases, the same hydrogen peroxide sensing equipment will be used for several different products and processes. Vaisala’s HPP270 series probes provide repeatable measurement, ideal for multiple processes, and are easy to calibrate on-site. Other life science applications that benefit from hydrogen peroxide vapor bio-decontamination include active pharmaceutical ingredient processing, drug compounding pharmacies, and distribution centers.
Today Cleamix delivers their units as standalone vaporizers or as networked modules for larger areas and ventilation systems. Their customers include bio-decontamination service providers, hospitals, military and defense organizations, agriculture and animal laboratories, and pharmaceutical manufacturers.
PDF
Learn more about Cleamix vaporizers at cleamix.com.
Learn more about Vaisala’s solutions for vaporized hydrogen peroxide measurement, monitoring and control.
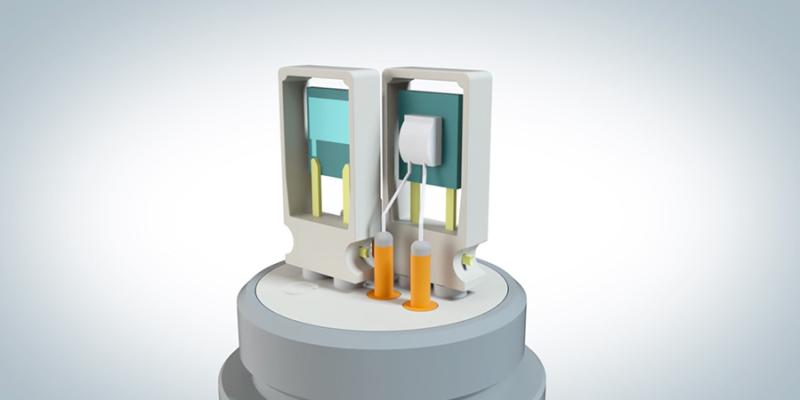
An in-depth look at Vaisala's hydrogen peroxide sensor technology

Vaporized Hydrogen Peroxide, Humidity and Temperature Measurement HPP270 Series
The Vaisala PEROXCAP® Hydrogen Peroxide, Humidity, and Temperature Probe HPP270 series probes are designed for demanding hydrogen peroxide bio-decontamination.
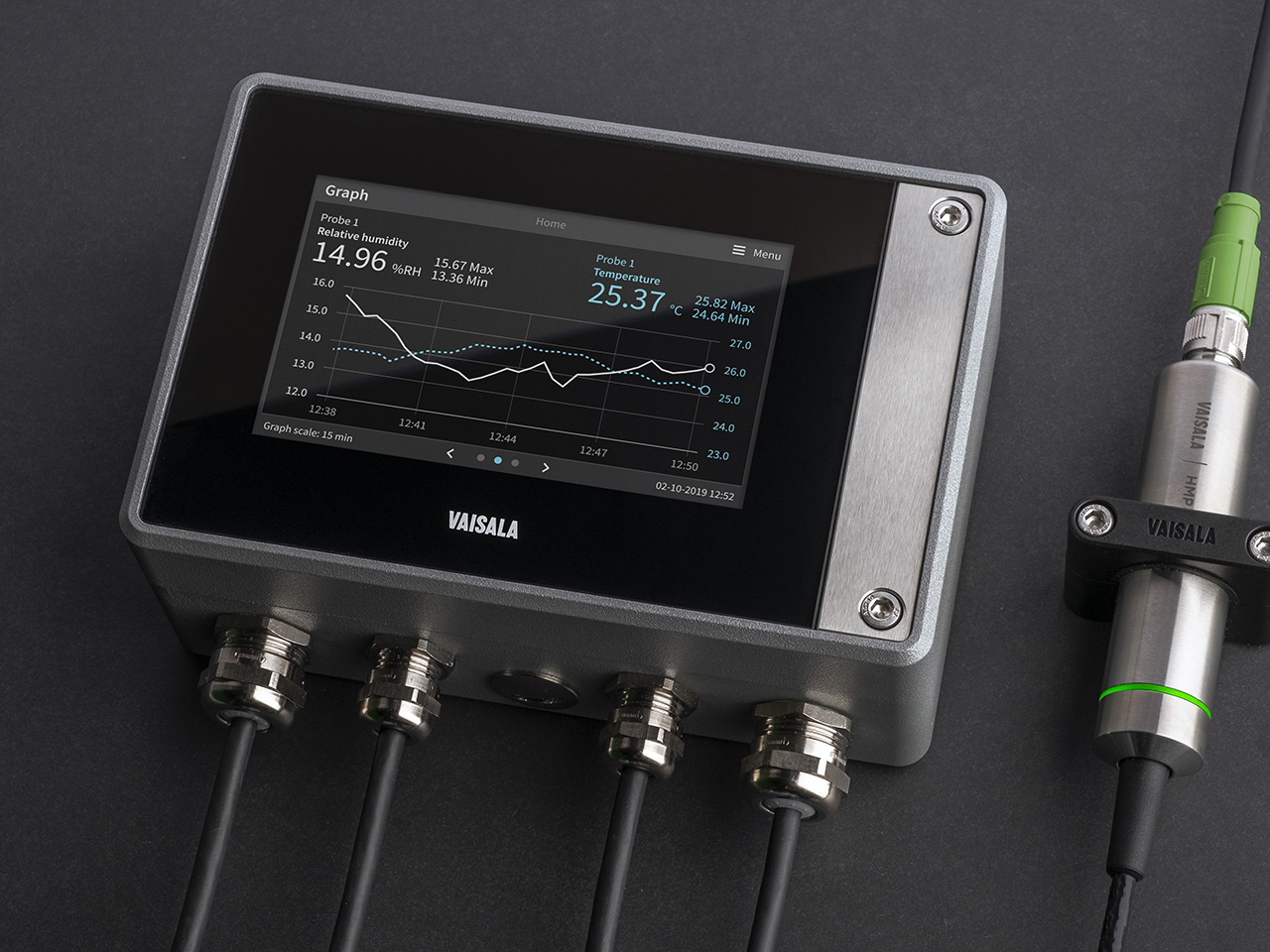
Indigo500 Series Transmitters
The Vaisala Indigo500 series transmitters are host devices for Vaisala Indigo-compatible, stand-alone smart probes. The Indigo500 series include multi-functional Indigo520 transmitter and Indigo510 transmitter with basic features.

Indigo200 Series Transmitters for Vaisala smart probes
Vaisala Indigo200 series transmitters are host devices for displaying measurement values from Vaisala's Smart Humidity, Temperature, Dew Point, Moisture in Oil, CO2 and H2O2 Probes.
Contact us
Our application engineers are ready to answer any of your questions.
On-demand webinar
From monitoring to controlling with vaporized hydrogen peroxide sensors: why, how & a case study
In this webinar, Vaisala’s sensor technology experts welcome two experts from Cleamix Oyj to explain how to integrate Vaisala’s sensors and transmitters and vapor generators to control vaporized hydrogen peroxide bio-decontamination applications.
Cleamix uses Vaisala’s HPP270 sensors to monitor and control the vapor generation with their portable vapor generators - the VCS-100 devices. Two Cleamix representatives will explain the advantages of integrated sensors for monitoring and controlling. Our Cleamix presenters will provide a case study of an effective bio-decontamination of a hospital room.
Two Vaisala sensor experts will also briefly describe Vaisala’s PEROXCAP® sensor technology and its unique ability to measure multiple parameters, including: hydrogen peroxide vapor, temperature, and humidity – as both relative saturation and relative humidity.