Temperature Mapping Worst-Case Scenarios in Refrigerators

Dear Vaisala,
Our Reply:
Articles on GxP standards & regulations
Visit our repository of articles, white papers, application notes and eBooks...
Comment
Janice Bennett-Livingston
Thank you for contacting Vaisala!
I have passed your request to our sales team... also available here: https://www.vaisala.com/en/contact-us

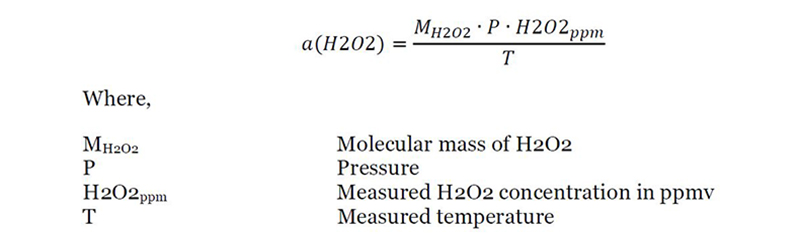










I need a system to register temperatures in order to qualify temperatures and humidity control Led áreas and equipements.
Please send me informativo about your offer about.
I offer qualification services for pharmaceutical companies.
Thanks.