Warehouse monitoring & mapping
For GDP-regulated storage & distribution
Reduce the risk of non-compliance and lost products in your life science holding and storage applications areas with the viewLinc Continuous Monitoring system:
• Easy connectivity - wired/PoE and wireless VaiNet data loggers provide connectivity for versatile installation
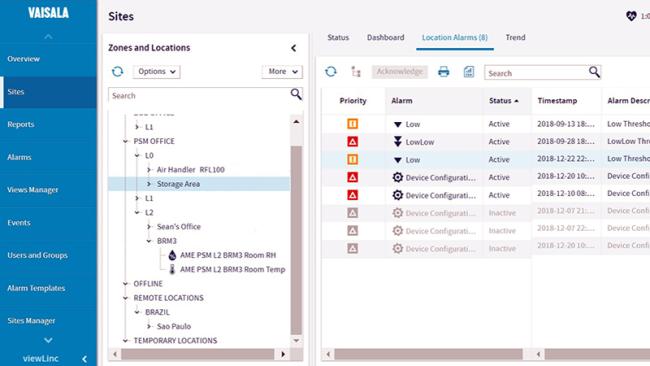
• 24/7 remote alarming via SMS, email, or local signal tower alerts
• Custom reports can be automated and delivered by email on demand, or scheduled
• Comprehensive IQOQ validation documents
• On-site installation/validation services available
• Indigo80 Hand-held indicators can be used for spot-checking and on-site calibrations
• Simplified warehouse mapping solutions. Save time and costs qualifying storage areas with Vaisala's Mapping kit
• Mapping services available in selected regions
• Choose from viewLinc Enterprise Server Software for on-premises or viewLinc Cloud.
For Commercial storage & distribution
Jade Smart Cloud monitoring system ensures the quality of stored goods, such as electronics, perishable goods, food & beverage, high-quality mechanical and aerospace products and other valuable items:
• Long product lifespan minimizes the total cost of ownership
• Long-term record of measurement data for reporting on storage conditions
• Easily scalable for a variety of storage applications
• Data securely stored in the cloud, reducing IT load
• 24/7 remote alarming via SMS & email
• Superior long-range LoRa® based VaiNet radio technology for reliable monitoring
• Easy addition of new loggers and access points enables effortless system expansion
• Accurate data based on Vaisala HUMICAP® proven sensor technology
• Indigo80 Hand-held indicators can be used for spot-checking and on-site calibrations
Warehouse monitoring and mapping products
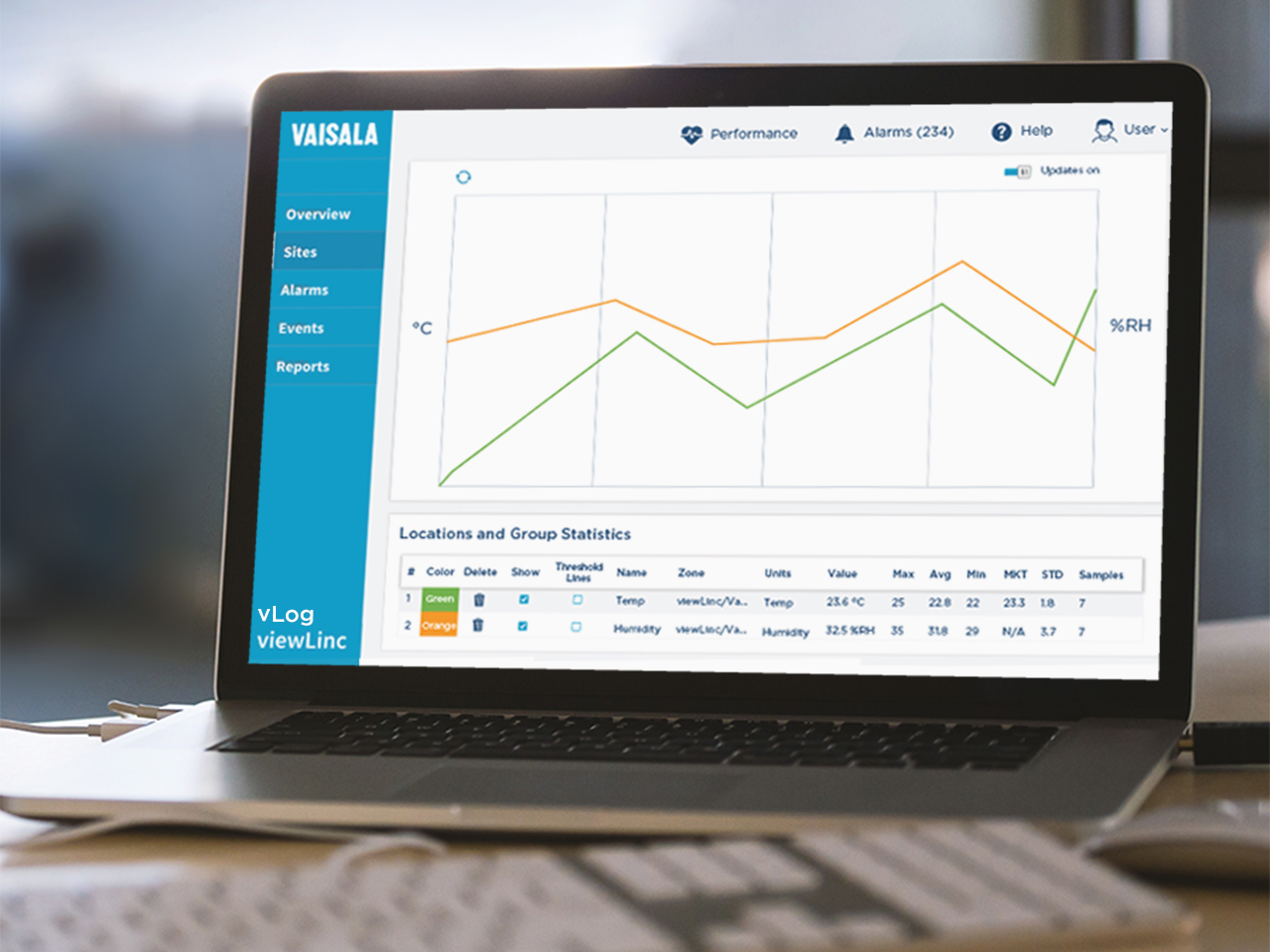
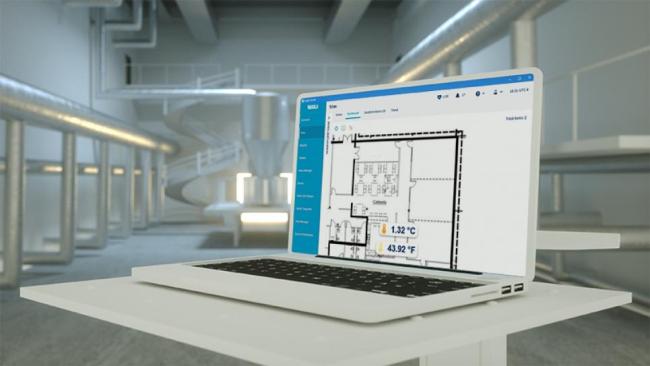
viewLinc Continuous Monitoring System (CMS)
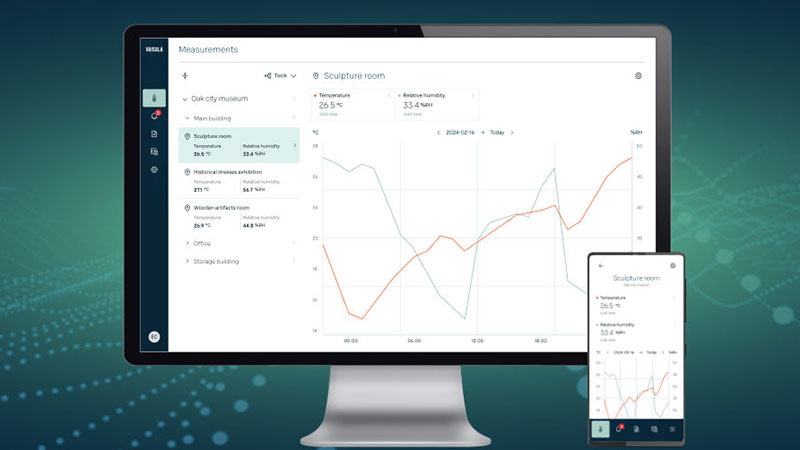
Jade Smart Cloud

VaiNet Wireless Temperature & Humidity Data Logger RFL100

VDL200 Data Logger

Validation mapping kit
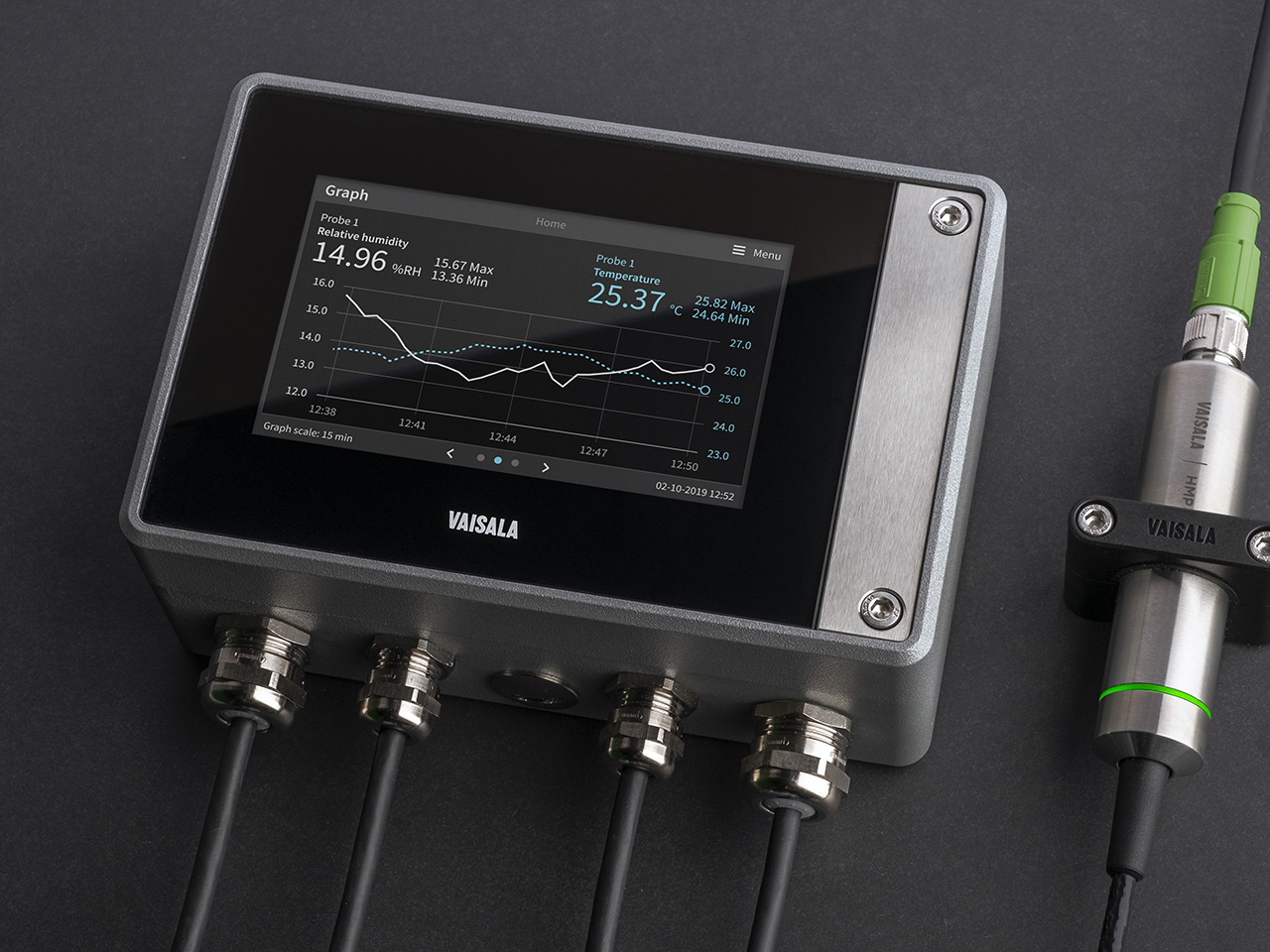
Indigo500 Series Transmitters

Indigo200 Series Transmitters for Vaisala smart probes

Temperature Transmitters TMT120/130

Temperature and Humidity Data Loggers DL2000

Temperature Data Loggers DL1016/1416

DL1000-1400 Temperature Logger