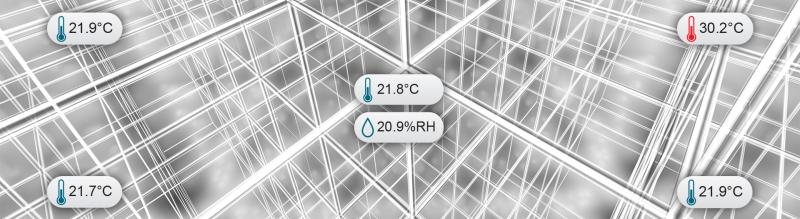
Mapping Best Practices for Controlled Environments
This learning module maps out each corner and everything in between when it comes to validation mapping studies. The Food and Drug Administration's (FDA) regulations tell us that we must identify the environmental conditions that can affect the strength, identity, safety, quality, and purity of our regulated products, whether they are pharmaceuticals, medical devices, or biological products. Get the job done right the first time with the following materials.
- Webinar: Mapping made easy, where to place sensors and why
- Application Note: 5 frequently asked questions on temperature & humidity mapping
- White Paper: Step-by-step guidelines for validating life science storage facilities
- Webinar: Continuous mapping for warehouses
- Application Note: Temperature mapping with viewLinc
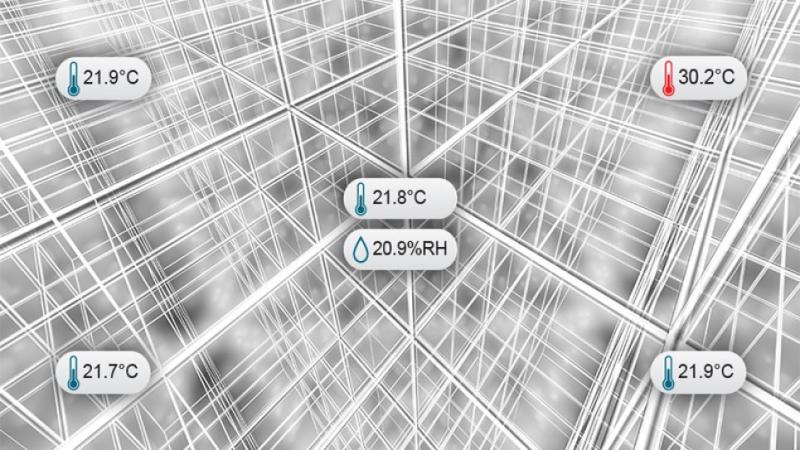
Mapping made easy: where to place sensors & why
This webinar will provide 5 simple rules for mapping studies to effectively determine sensor placement. Senior Regulatory Expert, Paul Daniel, will review the regulations and industry guidance on mapping along with discussing a validation method to reduce the total number of sensors required while maintaining your study’s defensibility under audit.
Topics:
- Review global regulatory changes and guidance on GDP.
- Learn techniques that ensure storage spaces meet specifications.
- Review crucial factors that impact sensor placement.
- Learn how to create an effective ratio of sensor to volume for mapping.
This webinar will include a Question & Answer period to elaborate on the topics.

Frequently Asked Questions about Temperature and Humidity Validation/Mapping
Whether you are an end user of a hired mapping contractor rest assured this application note will demystify the 5 most frequently asked questions regarding Validation/Mapping
- What limits should I use as an acceptable range for my study?
- What type of sensor(s) should I use?
- How many sensors do I need, and where should I place them?
- What kind of calibration do my sensors require?
- What is the appropriate duration for a mapping study?
Get your questions answered today.

GMP Warehouse Mapping Step-by-Step Guidelines for Validating Life Science Storage Facilities
Good manufacturing practice regulators have sharpened their focus on warehouse storage and distribution practices. Driving this trend is a shift in regulatory thinking, from quality-by-test to quality-by-design systems with an emphasis on level of risk to product quality and patient safety. This step-by-step guide describes how to map a warehouse to comply with internationally recognized GMPs .

Webinar: Continuous Mapping for Warehouses
Better Data, Better Compliance.
Have you ever wondered about leaving mapping sensors in place and using this high-density sensor deployment for monitoring?
This webinar is for you, we analyze the actual costs of continuous mapping, as compared to intermittent mapping studies. The results might surprise you, but the data quality and increase in environmental control is thought provoking.

White Paper: Temperature Mapping with viewLinc Monitoring System
Mapping with viewLinc
In this application note we outline how to perform mapping studies for room or chamber validation using viewLinc software with Vaisala data loggers.
CMS Products
viewLinc
A simple overview

viewLinc News
Explore all 5 of our Continuous Monitoring Learning Modules
Questions? Contact Our Continuous Monitoring Team.
We will respond within 24hrs.