Cooling crystallization monitoring and control in API production processes with RI measurements
This blog is second in a three-part series on how Vaisala refractometers can optimize API processes. See the first blog: “Perfecting solvent swap processes with Refractive Index trend data”.
Crystallization is an important method used in pharmaceutical manufacturing to purify, recover, and separate the active pharmaceutical ingredient (API) from a solvent. It involves the separation of the API as a pure solid form by cooling a solution (or slurry) containing the drug. Crystallization plays a crucial role in pharmaceutical processes, as most of the APIs are produced in a crystalline form. Moreover, crystallization has an impact on the quality and safety of the final drug product.
Crystallization control is important because it ensures that crystals with the desired properties, such as particle size distribution (PSD), are consistently produced. Properties such as PSD, in turn, play a critical role in the efficacy and stability of pharmaceutical products. For instance, the size of drug particles can affect their bioavailability, solubility, and rate of dissolution, which can impact their therapeutic effect. Any deviations from the cooling conditions will impact the product quality and downstream processability of the crystals.
Various techniques are used to control crystal quality and PSD, such as control of cooling rate, seeding, and supersaturation. Refractive index measurements provide selective concentration measurement of the mother liquor and a reliable measurement for following, in real time, supersaturation and for aiding crystallization control.
Achieving Controlled Crystallization with Supersaturation and Refractive Index Monitoring
Crystallization control through supersaturation monitoring is a common application for the Vaisala process refractometer. The aim is to aid crystallization and PSD control by monitoring the concentration of the mother liquid during pre-concentration and crystallization.
Supersaturation is the state in which the concentration of solutes in a solution exceeds its equilibrium saturation point, and it is a driving force for crystal growth and nucleation. In order to control crystallization and obtain good PSD, the concentration of the solution is ideally kept close to the solubility curve within the metastable zone. If the cooling rate is exceeded past the ideal point of saturation, the solution passes the super-solubility curve, entering an unstable zone where crystallization happens spontaneously and rapidly. Once this occurs, crystal sizes cannot be controlled. By controlling the concentration of solutes, it is possible to control the degree of supersaturation, which can affect the particle size distribution during crystallization.
In one of our customers’ cases, refractive index has been a useful tool from the early stage of development to study crystallization conditions and develop cooling crystallization profiles. In their tests, scientists used refractive index measurements to gain process understanding of the cooling crystallization of a certain API. They noted:
“The crystallization process is clearly visible in the refractometer data. As the liquid concentrates, the refractive index increases until it reaches saturation, then drops rapidly as the solute transitions from the liquid phase to the solid crystal phase. The exact onset of crystallization can be observed.”
Refractive index is a selective measurement of the liquid concentration, which makes it ideal for monitoring supersaturation during crystallization operations. Our customer concludes that refractive index values proved to be useful for achieving greater control over the PSD, showing high measurement reliability even in the presence of suspended solid material (during crystal formation) and gas bubbles.
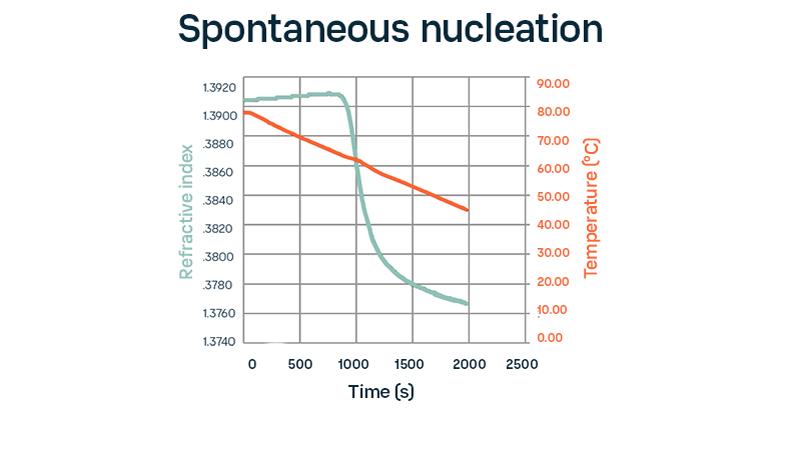
“Refractive index is a selective measurement of the liquid concentration, which makes it ideal to monitor supersaturation during crystallization operations.”
RI was also used by the customer to obtain relevant scientific data to study crystallization, such as solubility, helping them create solubility curves and crystallization profiles needed to determine the ideal crystallization conditions. Our customer explains that “when controlling by supersaturation, the goal is to achieve a saturation curve that closely aligns with the solubility curve. This indicates that crystallization has occurred in a more controlled manner, close to the metastable zone, and this is exactly what we can see with the process refractometer.”
The Vaisala Process Refractometer delivers continuous, real-time measurement, providing actionable insights that help control crystal quality and size, ultimately enhancing the quality and efficacy of the final drug product. Furthermore, effective crystallization control positively impacts downstream operations such as filtration. In our next blog, we will explore how refractive index measurement can be leveraged to design and optimize filter cake washing operations.





Add new comment