vH2O2 Vapor Concentration & Condensation Point
This blog is the fourth in a 4-part series where we describe how process parameters influence vaporized hydrogen peroxide bio-decontamination applications.
First blog: Humidity, condensation point, and maximum achievable vH2O2
Second blog: H2O2 Solution Concentration Ratio, Condensation Point & Maximum Achievable vH2O2
Third blog: Temperature, Condensation Point & Maximum Achievable vH2O2 ppm in Bio-decontamination
In this series, we propose four basic process parameter rules. This blog focuses on the 4th rule:
Increasing the H2O2 vapor concentration decreases the amount of water vapor the air can hold, and therefore, condensation occurs sooner.
In Figure 7 (from our white paper), we can see that increasing the H2O2 vapor concentration decreases the amount of water vapor the air can hold and condensation occurs sooner.
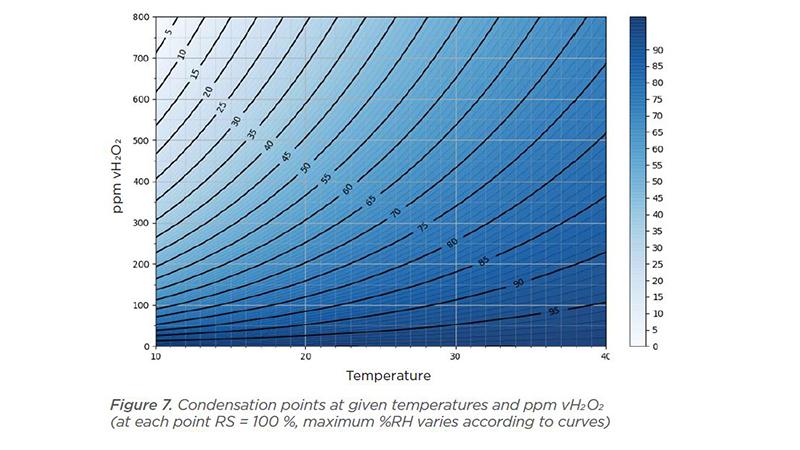
Each point in Figure 7 is a condensation point, meaning that Relative Saturation is 100 %. Temperature is on the x axis and ppm vH2O2 is on the y axis. The curve in the graphs shows the maximum relative humidity at a given temperature and concentration of ppm vH2O2.
As shown, at 20 °C and 300 ppm hydrogen peroxide, relative humidity will be 60% and relative saturation will be 100%. If we increase vH2O2 to 600 ppm at 20 °C, relative humidity is then 39 % and relative saturation is 100 %.
By increasing the air temperature to 40 °C with vH2O2 concentration at 300 ppm, relative humidity will be 87% and relative saturation will be 100%. The higher the temperature, the more water vapor the air can hold; relative humidity is increased.
To summarize our proposed rules for controlling condensation in vaporized hydrogen peroxide bio-decontamination applications:
- Lowering the initial level of humidity increases the amount of H2O2 vapor that can be used before condensation.
- Increasing the temperature increases the amount of water and hydrogen peroxide vapor the air can hold, thereby increasing the maximum achievable ppm vH2O2.
- A higher concentration H2O2 solution increases the H2O2 vapor that can be used before condensation.
- Increasing the H2O2 vapor concentration decreases the amount of water vapor the air can hold, and therefore, condensation occurs sooner
Conclusion
Over this series of blogs (based on our white paper), we have demonstrated how an in-depth understanding of the relation between critical process parameters allows for the development of effective, repeatable vH2O2 bio-decontamination cycles.
Single-parameter measurement is often insufficient for monitoring and ineffective for process control. We have also shown why relative saturation is an important value in accurately predicting condensation. This is why Vaisala’s unique PEROXCAP® technology measures multiple parameters in a single sensing unit, including: hydrogen peroxide vapor ppm, temperature, dew point, vapor pressure, and humidity as both relative humidity and relative saturation.
Please leave a comment in the fields below, or contact us if you have any questions!




Add new comment