Temperature Mapping Today: What’s Changed, What Hasn’t, & What Matters Most

In the world of regulated manufacturing, temperature mapping has long been a foundational validation tool—crucial for ensuring the safety and efficacy of pharmaceutical products, biologics, and medical devices. For those managing warehouses, labs, fridges, freezers, or cleanrooms in GxP-regulated environments, understanding the deeper role of mapping—not just as a checkbox activity, but as validation itself—is essential.
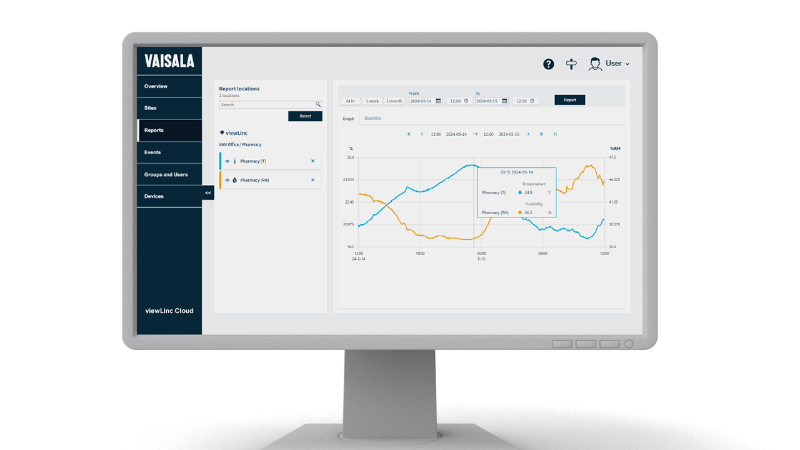
Mapping Is More Than Monitoring
Modern monitoring systems offer real-time data, automated alerts, and audit-ready records. It’s tempting to assume that this is enough. But monitoring and mapping serve very different roles. While monitoring ensures that a known system remains within spec, mapping proves that the system is capable of maintaining those specifications—across all locations and under real conditions. Mapping is validation. It qualifies a controlled temperature unit (CTU) or area by demonstrating uniformity and suitability for sensitive or high-value product storage.

Why Map?
If you haven’t performed a mapping study on your GxP-regulated application, you’re essentially gambling with your product. Yet time and time again, we receive this question from customers:
“Do I need to map if I’m monitoring with reliable, calibrated sensors?”
The short answer is: Yes, you do.
While there are certainly facilities that go straight to monitoring, skipping the mapping phase, that doesn’t make the approach compliant—or wise. Regulations, including those from FDA and EU GMP Annexes, clearly require temperature mapping as a prerequisite to environmental monitoring. And for good reason.
Mapping establishes the environmental profile of a space. It identifies how temperature (and other parameters like humidity or differential pressure) behaves over time and across different locations within a controlled area. Without this data, you’re essentially flying blind. You wouldn't know where the warmest or coldest points are—or how external conditions might affect internal stability.
While some industry professionals may downplay the idea of “hot spots” and “cold spots,” the fact remains: without mapping, you never know if or where they exist. Mapping provides the evidence needed to place monitoring sensors intelligently—where they’re most likely to capture meaningful data.
What if I (truly, honestly) Cannot Map?
It’s true that if forced to choose only one, monitoring would offer proof that at least one location in your space was in specification. But that’s a false choice. Mapping and monitoring serve different but complementary purposes. Skipping mapping isn’t just cutting corners—it’s compromising compliance and risking product quality.
Especially in Good Distribution Practice (GDP) environments, mapping is an area that deserves more attention. Investing in proper mapping up front leads to smarter monitoring and stronger compliance over the long term. Let’s look more closely at why mapping studies still matter.
Mapping in a Post-GDP World
The introduction of Good Distribution Practices (GDP) brought a seismic shift. Where temperature mapping was once seen as a best practice, it is now codified in regulations governing warehouses and distribution. Yet this expansion of responsibility often falls on facilities with limited budgets and less mature Quality Management Systems (QMS). These environments lack the procedural backbone that makes true validation possible. Mapping without a strong QMS risks producing data with limited meaning—or worse, misleading.
In such cases, implementing a validated monitoring system may provide more consistent quality control than a poorly executed mapping study. Still, for core GMP facilities, mapping remains irreplaceable. It ensures every cubic meter of your environment meets the storage specs you promise to your regulators and to your patients.
Equipment & Data Integrity
The most common pitfall in temperature mapping is skimping on equipment. Low-cost loggers may save money upfront, but they jeopardize study results. Mapping accuracy relies on high-quality, well-calibrated sensors—preferably RTDs over thermocouples due to their stability. You’ll also want data logging software with ample memory and software that streamlines analysis and reporting. From a regulatory standpoint, mapping data is how auditors assess your quality management. Fail to verify calibration? They’ll ask for your SOP. Miss a signature? They’ll question your document controls. Errors in mapping records often lead to deeper scrutiny.
Rethinking Remapping: From Routine to Risk-Based
Another area that is evolving is the frequency of remapping. Many organizations remap on fixed intervals—every year or three years—without assessing whether it’s truly necessary. A more strategic approach, encouraged by recent guidance updates, is to use risk assessments to determine when remapping is warranted. This helps avoid wasted effort and ensures your resources are focused where they make the greatest impact.
When to Map In-House & When to Outsource
Should you build your own mapping program or outsource? The decision comes down to two key factors: frequency and familiarity. If you perform temperature mapping regularly and have the tools and expertise, internal mapping can be efficient. But if you’re unsure how to select sensors, design protocols, or interpret results, bringing in a qualified service provider is often the smarter (and safer) choice. Observe the experts do it a few times, and build the skills to eventually take it in-house.

Game-changing technology
Modern innovations—like wireless data loggers with long-range secure transmission—are making warehouse mapping easier and safer. These tools allow real-time data visibility during qualification, so teams can detect issues and make adjustments before they invest the labor to retrieve loggers from 30 feet in the air. With fewer re-tests and less downtime, this tech reduces cost, improves outcomes, and eliminates surprises during audits.
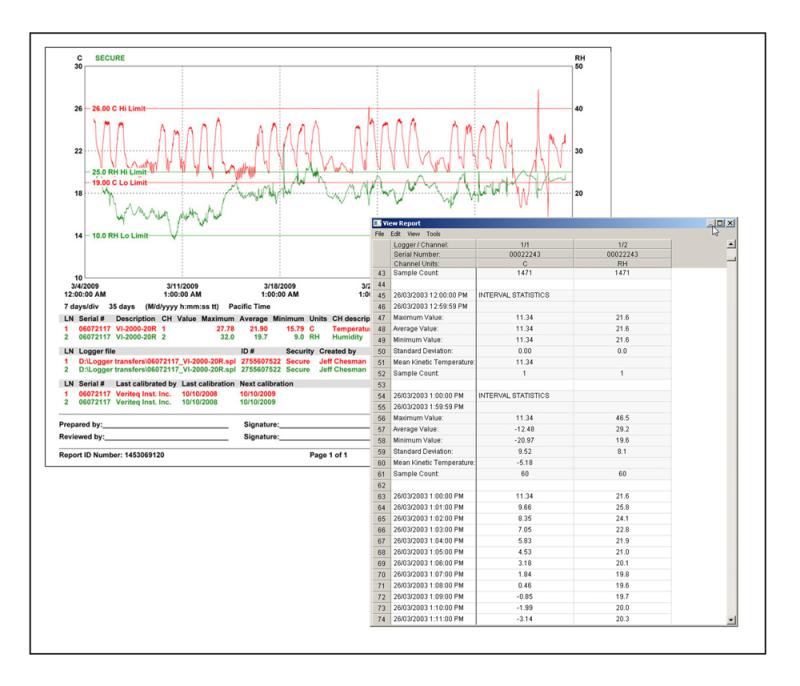
Final Advice: Embrace the Process, Expect Deviations
For any high-stakes or first-time mapping project, one piece of advice stands above the rest: Be patient. Mapping and validation take time, and trying to rush them guarantees stress, mistakes, or worse… noncompliance.
Accept that deviations will occur; in fact, they often reveal more about your process than a perfect run. A well-documented deviation shows you understand your system. A flawless run, by contrast, may raise questions if it's too good to be true.
Conclusion
Temperature mapping is required by regulation and whether you're managing a single blood bank fridge or an entire biologics warehouse, a thoughtful mapping strategy—supported by the right tools, people, and processes—is not just about compliance. It's a commitment to the safety of the patients who will ultimately use the products.
Resources

viewLinc Continuous Monitoring System

vLog Mapping/Validation system

Articles for GxP-Regulated Environments
This resource hub offers expert insights into validation, compliance, and best practices for managing pharmaceutical and life science environments. Topics include achieving 21 CFR Part 11 and Annex 11 compliance with environmental monitoring systems, applying GAMP methodologies for software validation, and using Mean Kinetic Temperature (MKT) in stability and distribution. Detailed guidance is provided for GMP warehouse mapping and aligning environmental monitoring with FDA/ICH expectations.

Support & Services
We provide a comprehensive suite of services to support every aspect of your environmental monitoring system—including software, sensors, and network connectivity components. From expert deployment to hands-on training, our Continuous Monitoring System (CMS) services equip your team with the knowledge and tools to operate confidently and compliantly. Whether you're installing a new system or optimizing an existing one, our services ensure your monitoring solution is efficient, reliable, and fully aligned with the most rigorous GxP and regulatory requirements.