Hospital environmental monitoring systems: What facility managers should know
In our recent webinar: "Monitor. Prove. Protect: Compliance in Healthcare Environments" I answered many questions from attendees. The answers are summarized below.
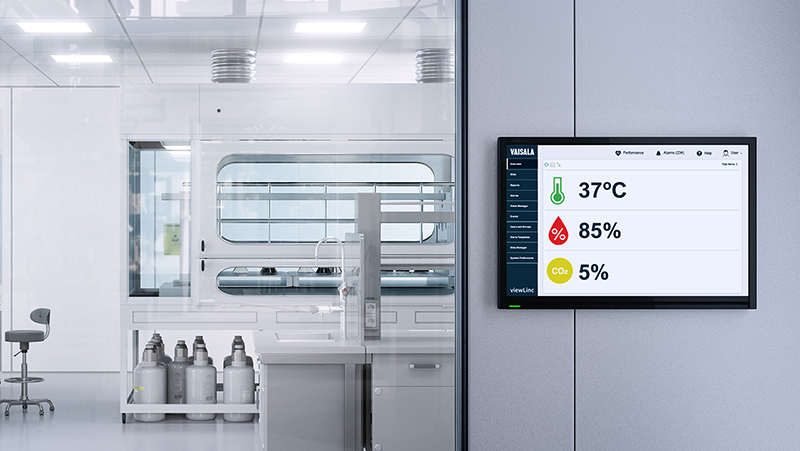
Hospitals and clinics depend on controlled environments to deliver safe medicine and therapies. A few degrees of temperature drift in a pharmacy refrigerator—or an undetected humidity spike in an operating suite—can mean failed surveys, lost product, or patient risk. That’s why so many healthcare facilities are moving to automated hospital temperature monitoring systems that track critical conditions in real time.
I’ve worked with hospital customers for years, helping them deploy monitoring systems that meet both regulatory expectations and the realities of complex healthcare environments. Here’s what I’ve learned about what facility managers really need to know when choosing and running a hospital environmental and temperature monitoring system.
Why hospitals stay with a monitoring Vendor
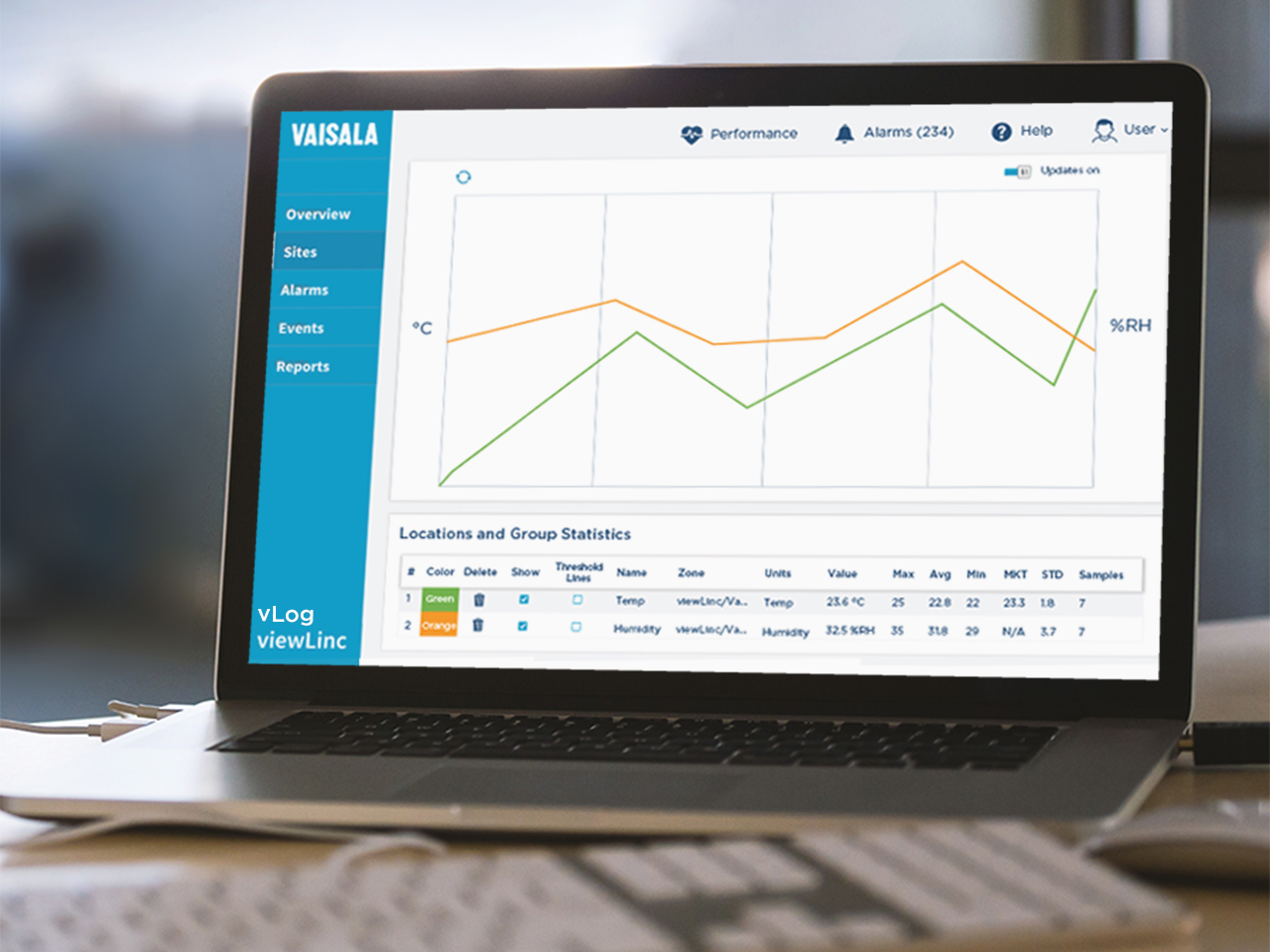
I can’t name our biggest customer, but I can tell you they’ve been with us for several years and in that time, they’ve standardized Vaisala’s viewLinc monitoring system across departments. They started small—in pharmacies and clinical research projects only. Later, they added their operating and procedure suites.
Over time, other departments saw the value of access to real-time environmental data and being able to prove compliance to surveyors. When a system works, word spreads.
Now they’re upgrading to our latest generation of VaiNet RFL100 data loggers, updating to the newest software version, and expanding onto a new hospital campus. That’s significant because an upgrade is the perfect time to switch vendors—but they didn’t. To me, that says the hospital temperature monitoring system delivered—and kept delivering—over the years.
Can monitoring make clinical research more efficient?
A good temperature monitoring system doesn’t just help with compliance—it can actually accelerate research. Clinical trials happen in three phases: first to prove safety, then efficacy, and finally superiority over existing treatments. Each phase can take years and often involves only a small group of patients.
If a batch of research drug is stored incorrectly, it can’t be used. When that happens, patients drop out of the study rather than waiting for a new batch to arrive. Lost product means lost patients, which means lost time to market. Reliable monitoring protects against that by ensuring drugs, blood, plasma, and other critical materials are stored within specification at every step.
How long does it take to deploy viewLinc?
It’s hard to predict every challenge in a large-scale deployment, but viewLinc Cloud was designed to make the process easier. There are no servers or software installations to manage. You install the hardware, turn it on, connect it to the Internet, and it automatically finds the cloud. You log in—and your controlled environments are monitored, data is secure, and medical products safeguarded.
How stable is the network connection?
Unstable networks are a common frustration, especially in large healthcare facilities. All Vaisala devices are data loggers that store data locally for at least 30 days. If the network goes down, the data is saved and automatically forwarded when connectivity is restored.
With VaiNet, all communication is encrypted and secure. Even if transmission is interrupted, there’s no distortion or data loss—critical in any hospital temperature monitoring system.
How does viewLinc handle network or power outages?
Power failures and network outages happen. But they don’t have to leave you with data gaps. Our RFL100 data loggers are either battery-powered or have battery backup, so they continue to log and alarm locally when needed. Once the network or power is restored, they send the stored data to viewLinc automatically.
Most hospital IT systems use Uninterruptible Power Supply (UPS). You’ve probably seen those red outlets on the walls—they’re there for life-safety systems that can’t lose power. The same logic applies to hospital monitoring systems that protect drugs, vaccines, and plasma storage.
How does viewLinc maintain an audit trail?
Data integrity is king in critical, regulated environments. However, you can’t demonstrate compliance without a reliable audit trail. In viewLinc, every configuration change is tracked automatically—who made it, when, what the old value was, and what the new one is. As an option, viewLinc can be set up to require users to enter a reason for each change. The key is that once data has been collected, it can’t be altered. That’s how we ensure trust in every report.
How do we manage alarm notifications across departments?
Alarm notifications in viewLinc are managed through templates and user groups. Each user is assigned to a group that has defined rights. If a group is linked to a specific alarm template, everyone in that group receives notifications when an alarm triggers.
Notifications can be sent by email, text, or even pager—yes, some hospital customers still use pagers. The system is flexible enough to work with whatever communication method your facility uses.
How does the system help in regulatory surveys?
viewLinc generates detailed reports that clearly show whether environmental conditions remained within defined tolerances. Our healthcare facility customers use these reports to demonstrate compliance during surveys by regulatory and accreditation bodies.
While requirements vary by region, the same principles apply everywhere: traceable data, complete audit trails, and clear documentation of environmental control. Our customers tell us that viewLinc’s hospital temperature monitoring system reports easily meet those expectations—whether the regulator is a national health authority, accreditation survey, or internal quality review.
Final thoughts
Monitoring isn’t just about passing surveys—it’s about knowing the system will work when you need it, sending alarms to the right personnel, in time to safeguard product. A good hospital temperature monitoring system should make compliance easy, protect valuable materials, free your staff from manual checks, and eliminate IT headaches.









Add new comment