Monitor. Prove. Protect: Compliance & Care in Healthcare Environments
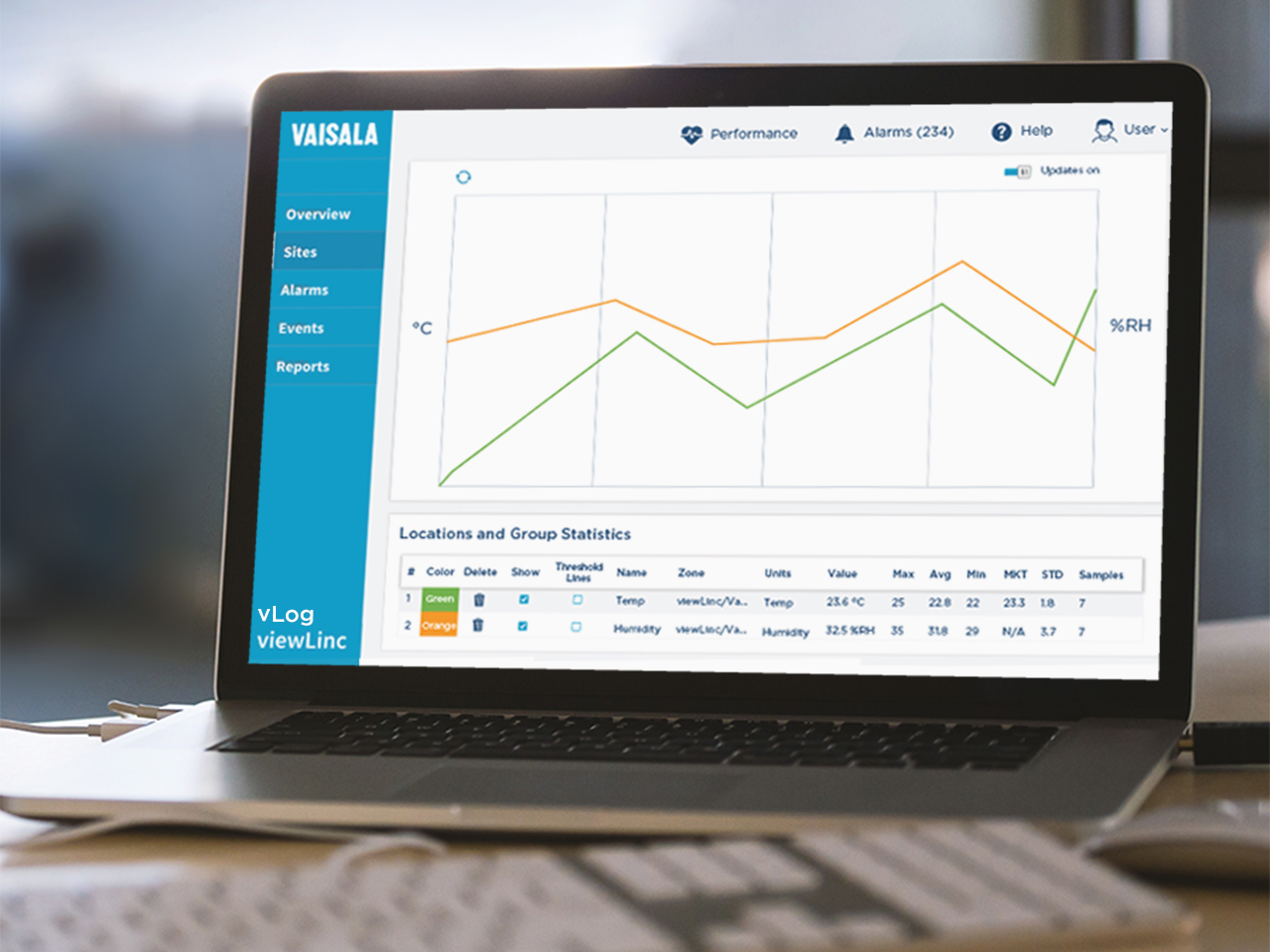
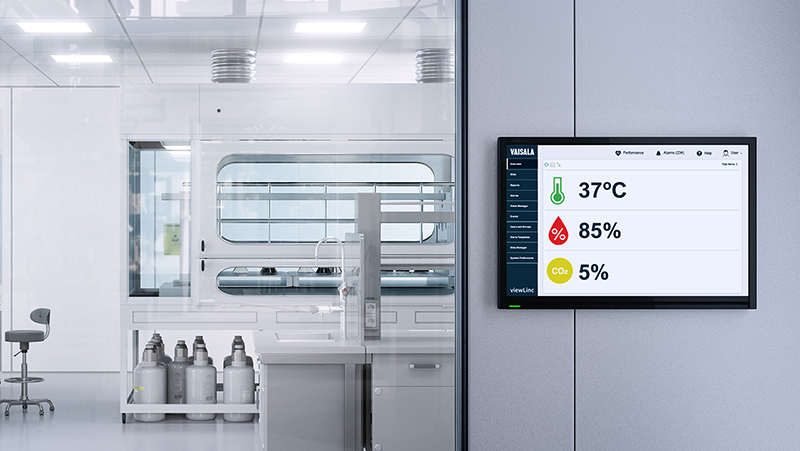
In hospitals and clinical settings, environmental parameters like temperature, humidity, pressure, and CO₂ directly affect patient safety, clinical workflows, and regulatory compliance. In this webinar, Vaisala’s Senior GxP Regulatory Expert shows what a fit-for-purpose continuous monitoring system looks like across pharmacies, OR suites, quarantine rooms, labs, and research drug storage.