Hospital Pharmacy Monitoring
Of the FDA's Top 10 Observations in 503B Pharmacies, the second most common observation (69.8%) of was:
"Aseptic processing areas are deficient regarding the system for monitoring environmental conditions."
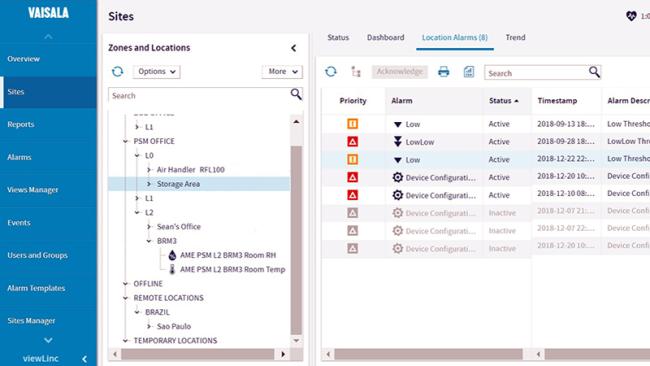
Reduce the risk of lost product and regulatory non-compliance in your controlled environments, pharmacies, ORs & procedure rooms, blood and tissue banks, and labs.
viewLinc Cloud monitoring system is built for compliance with VFC JCAHO, CAP, 21 CFR Part 11, ISO 17025, and GxP regulations.

Protect Products & Patients
- Validated, GxP-Compliant Cloud System – Purpose-built for regulated life-science environments, including hospital pharmacies, blood and tissue storage, and labs; meets 21 CFR Part 11 and Annex 11 requirements.
- Minimal IT Burden – As a SaaS platform, viewLinc Cloud eliminates on-premises servers and reduces IT maintenance while ensuring continuous monitoring, alarming, and reporting.
- Scalability & Accessibility – Supports anywhere from a single fridge to thousands of sensors across sites, accessible from any web-enabled device, with 7-year data retention.
- Seamless Integration with Vaisala VaiNet Hardware – Wireless RFL100 loggers (for temperature, humidity, CO₂, incubators) and AP10 access points offer flexible, reliable coverage.
Data Integrity & Regulatory Confidence in Every Measurement
- End-to-End Data Security – Multi-layer protection with encryption in transit and at rest, multi-factor authentication, role-based access, and third-party security audits.
- Data Integrity – Gap-free logging, on-device memory & backup during outages, tamper-proof databases, and encrypted transmission and storage.
- Multi-Layer Security & Access Control – Role-based access, multi-factor authentication, and controlled user permissions.
- Automated Validation & Update Transparency – Built-in validation testing, visible validation status, and documented change-control processes for each software update.
- Continuous Validation & Controlled Updates – Background validation, pre-release testing, changelogs, and risk assessments for every version.
"Of all the monitoring systems we looked at, the viewLinc monitoring system provided the best value ... hands down!"
- Dorraine Reynolds, Pharmacy Director US-based National Research Hospital
See more customer stories from blood, tissue, IVF, clinical research, pharmacy and hospitals
Hospital Pharmacy Monitoring Related Products
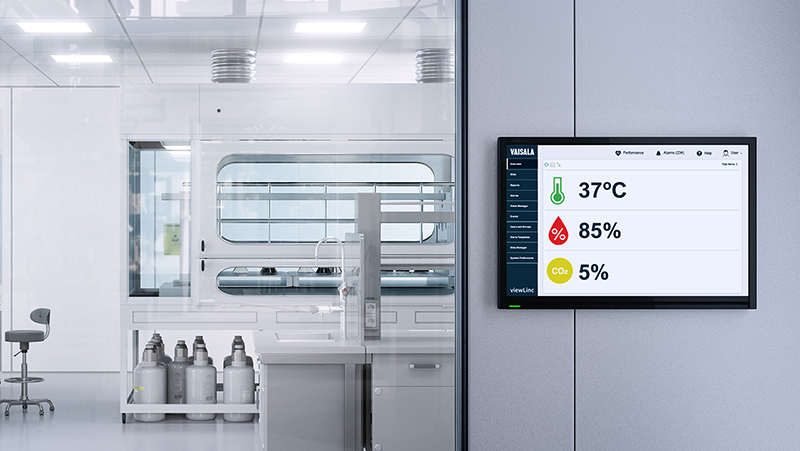
viewLinc Cloud
viewLinc Cloud monitors controlled environments in life science. The system saves IT resources, scales easily, provides superior security, and ensures system availability.

VaiNet Wireless Temperature Data Logger RFL100
The RFL series data loggers use Vaisala’s LoRa® based VaiNet wireless technology to monitor temperature in fridges, freezers, incubators, LN2 tanks, cold rooms, and very low temperature freezers.

VaiNet Wireless Temperature & Humidity Data Logger RFL100
The RFL100 data loggers use Vaisala’s proprietary VaiNet wireless technology to monitor environments ranging from warehouses, to production areas, to cleanrooms and laboratories

VaiNet wireless CO2 data logger RFL100
The RFL series data loggers use Vaisala’s LoRa® based VaiNet wireless technology to monitor CO 2 in incubators and chambers.

Temperature Transmitters TMT120/130
Temperature transmitters TMT120 and TMT130 offer accurate, reliable measurement capability with easily exchangeable probe. For demanding HVAC applications