Your Questions on VH2O2 Calculations Answered
Question: Is trending available that can be saved for later review?
Yes. We have two options for creating trend data with the HPP270 series probes: The first method is by connecting the probe to an Indigo520 transmitter, which includes trend display, data logging, as well as advanced trend data capabilities with the ability to export data to .CSV file. The second method is connecting the probe to Vaisala’s free Insight PC software. This allows you to record up to 48 hours of data from two parameters and export this data to a CSV-file.
Question: What is the maximum distance the temperature probe should be apart from the PEROXCAP® sensor?
The distance is not relevant so long as you have the temperature sensor in representative ambient conditions and the PEROXCAP® sensor in a representative gas mixture. For example, in still air conditions, you should avoid installing the temperature sensor on top of the PEROXCAP sensor, which gives off a tiny bit of heat. Install the temperature sensor where it will not be effected by heat sources, including the PEROXCAP probe. You can find more information on this topic in the HPP270 probe User manual: Section 4 Installation.
Question: Can the HPP270 probe be integrated with isolators for sterility testing?
Yes. Isolators are one of the most common use cases for the HPP270 series probes. Here is an application note that you might find interesting: Vaporized Hydrogen Peroxide Bio-decontamination in Isolators, cRABs, and Transfer Hatches.
Question: Can this probe be used as a controlling sensor to run a vapor generator to keep the relative saturation around 95% in a closed loop, 4 m3?
“Fighting superbugs proactively: innovation and collaboration in vaporized hydrogen peroxide bio-decontamination”
Question: Can the HPP272 withstand vacuum conditions?
The HPP270 series probes are designed for normal atmospheric pressure only. It is likely that the probe could withstand over and under pressure, but those conditions may have an effect on the measurement accuracy. In our estimation, the probe withstands +/-0.5 bar from the normal atmospheric pressure at least (= 0.5 bar to 1.5 bar). However, we have not tested it and therefore we do not specify over/under normal atmospheric pressure.
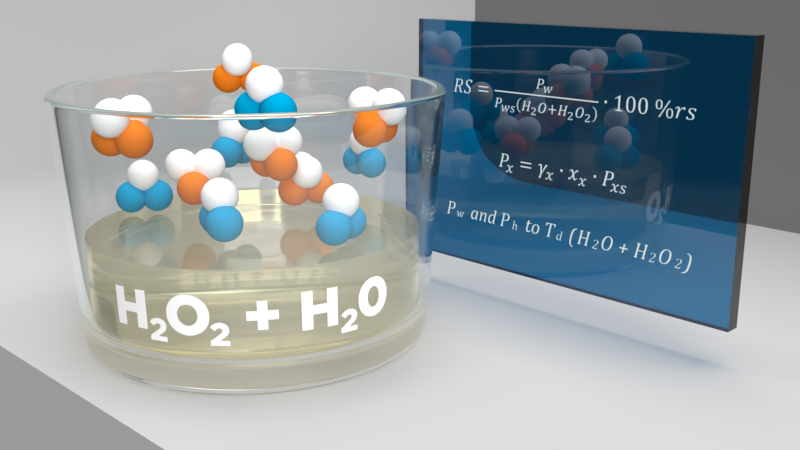
Question: Is there a simple formula for calculating the saturation point and vapor pressure of vaporized H2O2 in a closed circulation system?
Then, you can calculate vapor pressure of H2O2. The saturation vapor pressure of H2O2 is also presented in this same eBook; it can be calculated using equation 6.
Question: Has anyone developed formulas that determine the time it takes to reach equilibrium in a free evaporation environment based on a set of initial conditions like: stable temperature, surface area of the liquid, and gas volume of the environment?
Question: Once hydrogen peroxide is in vapor form in a closed environment, how fast does the vapor obtain a uniform concentration within the environment?

Add new comment