Enhancing antibody production with inline process refractometers


Minimizing human error & enabling continuous monitoring data
In today’s biopharmaceutical manufacturing—particularly in antibody production—process control, compliance, and reliability are essential. Even minor deviations can compromise yield, delay batch release, and risk regulatory noncompliance.
Vaisala PolarisTM inline process refractometers deliver real-time, liquid concentration measurements that automate control and inline sampling and provide accurate, continuous data for critical stages of the production cycle.

Raw material & feed solution preparation
Challenge:
Cell growth and productivity depend on precisely prepared feed solutions. Manual sampling and offline checks slow processes and risk error.
Polaris Solution:
Install refractometers directly on feed preparation tanks to monitor glucose, nutrients, and other key components in real time. Real-time feed verification prevents over/underfeeding.
Example: Verify glucose feed concentration before transfer to bioreactors.
Benefits:
- Eliminates manual sampling and lab delays
- Prevents nutrient imbalance
- Digital integration supports traceable batch records/QMS.
- The PR53AP 170 mm probe withstands high temperatures
- Flexible installation directly to kettles, cookers, vessels, and tanks.

Buffer preparation
Challenge:
Chromatography and filtration depend on precise buffer composition. Deviations can cause costly downstream failures.
Polaris Solution:
Monitor buffer concentration inline to confirm each batch meets specifications before use.
Example: Detect deviations during buffer make-up, allowing immediate correction.
Benefits:
- Improves process robustness and buffer consistency
- Simplifies compliance with 21 CFR Part 11
- Reduces operator workload and transcription errors

High-protein concentration monitoring in downstream purification
Challenge:
During ultrafiltration/diafiltration, protein concentration rises from dilute to highly concentrated levels—requiring precise control to avoid yield loss.
Polaris Solution:
Deliver continuous, drift-free measurement to trigger automated process steps when target concentration is reached.
Benefits:
- Enables real-time process automation
- Improves yield without manual intervention
- Maintains accuracy; no drift correction or recalibration
- Fully supports PAT/QbD strategies
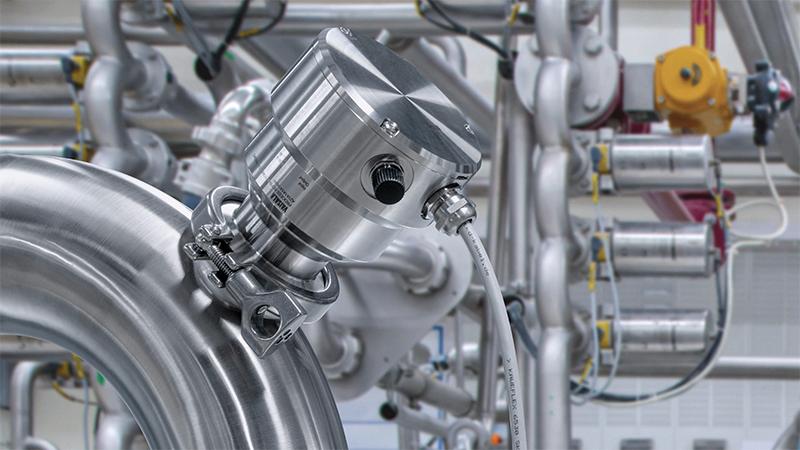
Formulation & final fill
Challenge:
Excipient concentrations must be exact to ensure stability, compliance, and batch consistency.
Polaris Solution:
Verify excipient levels inline during bulk mixing for immediate confirmation before filling.
Benefits:
- Enables Real-Time Release Testing (RTRT)
- Prevents out-of-specification batches
- Supports fully digital, audit-ready documentation

Key advantages of Vaisala Polaris refractometers
- High accuracy, even in concentrated proteins, salts, solvents, surfactants, and organic buffers
- Pharma-grade electropolished stainless steel with surface roughness < 0.38 μm (15 μin)
- Pharma-grade process connections: 1.5″ to 4″ Tri-Clamp or NovaSept®
- GxP support documentation including Installation and Operational Qualification (IQ/OQ) considerations
- Drift-free, maintenance-free, and no recalibration required
- SIP/CIP compatible; aseptic processing ready
- Continuous real-time diagnostics enabling instant detection of prism fouling
- FDA 21 CFR Part 11 compliant data logging
- Pre-stored chemical curves for fast product changeover
- Seamless integration with MES, SCADA, and automation platforms
Conclusion
Vaisala Polaris refractometers are purpose-built for the demands of modern bioprocessing. From Manufacturing and Process Development to Quality Assurance, they enable higher yields, greater compliance confidence, and faster batch release by minimizing human error and ensuring continuous, validated process data. Integrated into a PAT framework, Polaris refractometers help transform measurement into a competitive advantage.

Vaisala Polaris™ PR53AC Sanitary Compact Process Refractometer
The Polaris™ PR53AC sanitary process refractometer provides accurate, drift-free inline liquid concentration measurement (0–100%) for pharmaceutical and biotechnology applications. 3-A Sanitary/EHEDG certified, CIP/SIP ready, low-maintenance, and durable, the PR53AC supports quality, compliance, and efficiency in regulated manufacturing. Designed for pipeline installation.
The Polaris™ PR53AP has the same features as the PR53AC and is built to withstand high heat, mixers, scrapers, and sticky conditions, the PR53AP ensures safe, reliable performance and efficiency in demanding processing environments. With a 170 mm probe, PR53AP is designed for tank/vessel installations.