Refractive Index Measurement for Real-Time Protein Concentration Control in Blood Plasma Ultrafiltration
A Reliable measurement for optimizing protein recovery in plasma ultrafiltration
Introduction
Blood plasma contains hundreds of proteins that perform essential physiological functions. The most abundant, albumin and immunoglobulin G (IgG), make up roughly 80 % of plasma proteins.
Plasma is used as a raw material for producing pharmaceutical fractionated products such as human serum albumin (HSA), immunoglobulin G (IgG), thrombin subclass proteins, and protease inhibitors. These products are critical in treating life-threatening conditions including bleeding and clotting disorders, immunological and infectious diseases, and tissue degeneration.
Blood plasma can be fractionated from whole blood by centrifugation or obtained by apheresis. During collection, processing, and storage, strict control of environmental and process conditions—especially temperature—is vital to preserve plasma quality.
The plasma fractionation process begins with conditioning and pooling frozen plasma, followed by cryoprecipitation and subsequent fractionation steps such as cold ethanol precipitation, filtration, and chromatography. Each intermediate fraction proceeds to further purification before packaging and release.
Application
Measured Medium
- Blood plasma intermediate
Typical End Products
- Albumin
- Immunoglobulin G
Separation of albumin or IgG from human plasma intermediates is typically a small-scale centrifugal ultrafiltration batch process, whereas animal plasma is often processed in larger continuous ultrafiltration systems.
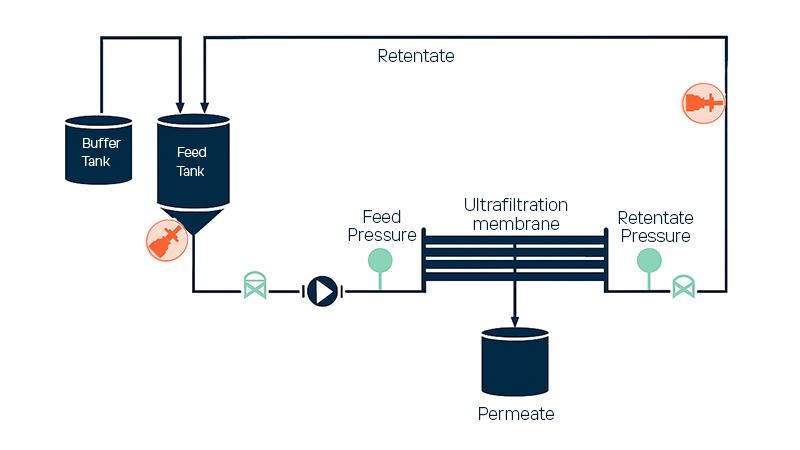
In both cases, the plasma fraction solution from earlier processing stages is pumped through membranes under pressure. Smaller molecules pass through the membrane, while larger proteins remain as the concentrated retentate. In continuous systems, buffer (e.g., 0.9 % NaCl) is added to maintain a constant volume while smaller molecules are removed.
Controlling the retentate concentration is critical—it affects membrane permeability, overall yield, and end-product quality. Precise measurement ensures compliance with labeled specifications and optimizes operating efficiency.
Instrumentation and Installation
The Vaisala Polaris™ PR53AC Sanitary Compact Process Refractometer provides real-time, in-line concentration measurement of plasma proteins during ultrafiltration. Continuous monitoring of refractive index enables process engineers to maintain target concentration levels accurately, improving consistency and throughput. Measuring refractive index in both feed and retentate streams allows compensation for buffer composition variations, ensuring simple and accurate protein concentration control.
As a Process Analytical Technology (PAT) tool, the refractometer replaces slow, sample-based Kjeldahl or Dumas nitrogen determination methods. Unlike spectrophotometric A280 measurements, which are limited by absorbance range, the Vaisala Polaris™ PR53AC can measure high-concentration protein solutions—up to and beyond 220 g/L—with excellent repeatability. This allows for direct, continuous measurement without the need for manual sampling or offline analysis, streamlining workflows and reducing the risk of error.
Typical Installations
- Direct angle mounting on the outer radius of a pipe bend
- Integration via a flow cell
- Direct insertion in the feed tank
The instrument outputs 4–20 mA and Ethernet signals for connection to data acquisition or control systems, supporting real-time process monitoring and documentation.
Because of the Polaris™ optical measurement principle, the PR53AC Or PR53AP deliver stable readings even in the presence of bubbles or suspended solids, requires no recalibration, and can be easily verified with standard refractive index liquids. The design supports CIP cleaning, minimizing maintenance requirements. A longer probe option is the Vaisala Polaris™ PR53AP Sanitary Probe Process Refractometer.
Instrumentation Description
- Measurement Range: Refractive Index (nD) 1.3200 – 1.5300 (0–100 Brix)
- Sanitary Design: Meets 3-A and EHEDG requirements; electropolished wetted parts (Ra 0.38 µm / 15 µin)
- User Interface Options: Multichannel MI, Compact CI, or Web-based WI interfaces for measurement and diagnostics access
- Verification: Factory calibrated; simple verification using standard RI liquids
Conclusion
The Vaisala Polaris™ inline process refractometers delivers accurate, real-time monitoring of protein concentration during blood plasma ultrafiltration. Its robust optical design and hygienic compliance make it ideal for pharmaceutical and biotechnology environments, enabling reliable control of product concentration, improved process efficiency, and compliance with GMP and PAT guidelines.

Vaisala Polaris™ PR53AC Sanitary Compact Process Refractometer
The Vaisala Polaris™ PR53AC provides accurate, real-time refractive index (RI) measurement for controlling protein concentration in blood plasma ultrafiltration. Its 3-A Sanitary and EHEDG-certified design ensures full compliance with hygienic manufacturing standards used in pharmaceutical and biotechnology processes.
The PR53AC delivers drift-free, in-line measurements unaffected by bubbles, color, or suspended particles, enabling stable process control and consistent end-product quality. Designed for CIP/SIP compatibility and easy installation on sanitary fittings or flow cells, it requires minimal maintenance and operates reliably across a wide concentration and temperature range.
This compact, durable refractometer supports mA, HART, and Modbus RTU connectivity, integrating seamlessly into automated GMP environments for efficient, continuous plasma fractionation monitoring.