Expert Article
Oil moisture expressed as water activity (aw)

Every fluid has the ability to hold a certain amount of dissolved water. The maximum amount of water that a given fluid can contain in solution is referred to as its saturation point. Once the fluid has reached its saturation point, any additional water introduced will separate out as free water by forming a distinct layer. Since most oils are less dense than water, the water layer will usually settle below the oil.
An oil’s saturation point is a function of many different factors such as the composition of its base stock (mineral or synthetic) as well as the type of additives present. Aside from these initial composition differences, the saturation point of an oil will vary over its lifetime as a working fluid. Two major factors that influence an oil’s saturation point as it ages are fluctuations in temperature as well as changes in chemical make-up due to the formation of new substances produced as byproducts of chemical reactions taking place within a dynamic oil system.
The traditional unit of measure for quantifying water content in oil has been ppm (parts per million). What is the significance of a ppm measurement? By definition ppm is an absolute moisture parameter that describes the volume or mass ratio of water to oil:
By volume: 1 ppm(v)water= 1 ml of water / 1 m3 of oil
or
By mass: 1 ppm(v)water= 1 g of water / 1000 kg of oil
By actively measuring ppm levels of water in oil, the absolute amount of water can be determined. However, a ppm measurement has one major limitation – it does not account for any variation in an oil’s saturation point. In other words, in a dynamic oil system with a fluctuating saturation point, a ppm measurement would provide no indication of how close the moisture level is to the oil’s saturation point. This becomes even more critical when the water content nears the oil’s saturation point, creating a risk of actually exceeding the saturation point and forming free water – a destructive contaminant to almost all oil applications.
To illustrate this concept, consider the following oil that undergoes a 40°C reduction in temperature:
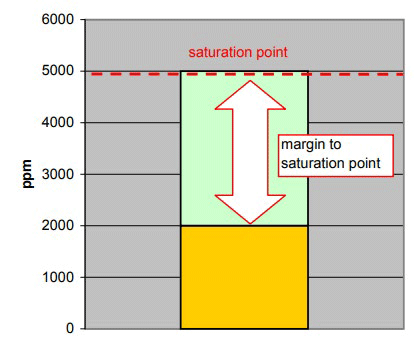
| Figure: Gearcase Lubricating Oil Temperature: 70 °C Saturation point: 5000 ppm Actual amount of water in the oil: 2000 ppm aw: ~0.40 |
The illustration shows that the saturation point of the oil at 70°C is 5000 ppm. The amount of water in this oil is 2000 ppm. This means that the oil can hold another 3000 ppm more water before the oil becomes saturated. This is sometimes referred to as the “margin” to the saturation point.
When the temperature of this oil drops to 30°C, the saturation point of the oil also drops to 3000 ppm. Note that the amount of water in the oil has not changed (still 2000 ppm). However, the margin to the saturation point has been reduced to 1000 ppm.
In this scenario, if an operator were only measuring ppm, he would see no change in the amount of water present (2000 ppm) even though the margin has been dramatically reduced and the saturation point has moved much closer to the water content, creating a greater risk of free water formation.
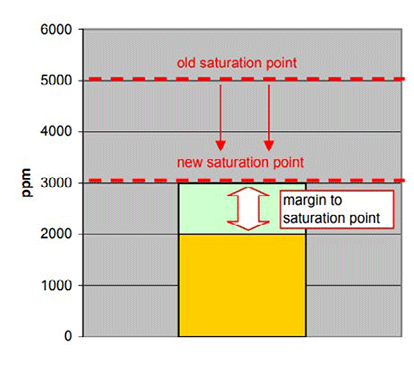
| Figure: Gearcase Lubricating Oil Temperature: 30 °C Saturation point: 3000 ppm Actual amount of water in the oil: 2000 ppm aw: ~0.67 |
What would happen if after one year, due to aging of the oil, the saturation point was further reduced to 1500 ppm? In this scenario, there is no longer a margin to saturation since the water content is now greater than the saturation point.
As before, an operator would continue to read a moisture content of 2000 ppm despite the fact that the saturation point has now been reduced to 1500 ppm resulting in 500 ppm of free water formation.
By measuring water activity instead of ppm, the above problems can be avoided.
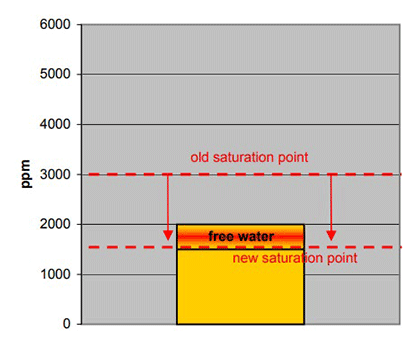
| Figure: Old saturation point Free water New saturation point |
What is water activity (aw)?
Water activity is the amount of water in a substance relative to the total amount of water it can hold. It is defined as:
aw = p / p0
where
p = the partial pressure of water in a substance above the material
p0= the saturated vapor pressure of pure water at the same temperature
In the example above, aw changes as a function of the saturation point (p / p0, the denominator). aw will also change as a function of actual water content in the oil, that is, water entering or leaving the oil. In other words, aw will always provide a true indication of the margin to saturation point.
While it is possible to derive a correlation between aw and ppm for any oil, the validity of this relationship over its lifetime in a dynamic oil system, for example in lubricating oil, will diminish. As discussed earlier, with age, a fluid undergoes changes in composition due to chemical reactions taking place which not only affect its saturation point but also its relationship to aw.
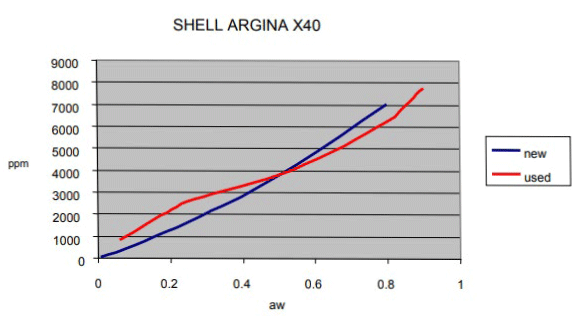
This graph(below), generated using test data from marine engine oil, compares the difference between new and used oil. Because of the continuous migration in the relationship between aw and ppm due to aging, it is difficult to maintain a valid correlation over an oil’s lifetime.
While there are many different methods of measuring moisture in oil available in today’s market, the latest in-line water activity measurement technology uses a capacitive type sensor that operates on an absorption principle.
The sensor is a capacitor consisting of an upper and lower electrode with an insulating material in between known as a dielectric. The dielectric absorbs and desorbs water molecules, changing the dielectric constant and thereby the capacitance of the sensor. Water absorption is proportional to water activity of the fluid. The benefits associated with this type of technology are the ability for direct in-line installation, a very fast response time, and good chemical durability suitable for a wide range of fluids.
Good candidates for this in-line technology include applications involving large oil or hydraulic systems such as paper machine lubrication, turbine and transformer operation, and oil reclamation system manufacturers. With many manufacturing facilities today employing some type of predictive maintenance program designed to prevent machine downtime and extend equipment life, an in-line, continuous moisture measurement becomes an integral part of this fluid management plan.
In conclusion, while the traditional unit of measure for expressing moisture content in fluids has been ppm, measuring aw can offer a more complete picture of the condition of a fluid:
- Regardless of the saturation point of the fluid, an aw reading will always provide a true indication of risk of free water formation.
- As the saturation point increases or decreases for whatever reason (e.g., temperature, age, change in physical properties), aw accurately reflects the new margin to saturation.
- aw is independent of the fluid being measured. Since aw applies to all fluids and solids, it can be used universally for all substances regardless of chemical composition or physical characteristics.