Pure Water Treatment by Chemical Precipitation
Introduction
Pure water treatment is the process of removing undesirable chemicals, biological contaminants, suspended solids and gases from raw water. Water purification aims to produce water for a specific purpose, for example, for human consumption and medical or industrial use.
Polyaluminium coagulants are finding increasing use in potable water treatment plants, particularly, for soft, colored surface waters. Polyaluminium chloride (PACl) is gradually replacing the Alum (aluminium sulphate), a commonly used coagulant in water treatment plants. Alum coagulates at a limited pH range (between 5.5 an 6.5), and often requires the addition of alkali to the raw water to achieve the optimum coagulation pH. Furthermore, the alum floc produced is particularly fragile. This is especially important if a coagulant is required to maximize color removal in a microfiltrationbased water treatment process.
Typical end products
- Drinking water
- Medical, pharmacological, chemical and industrial water applications
Application
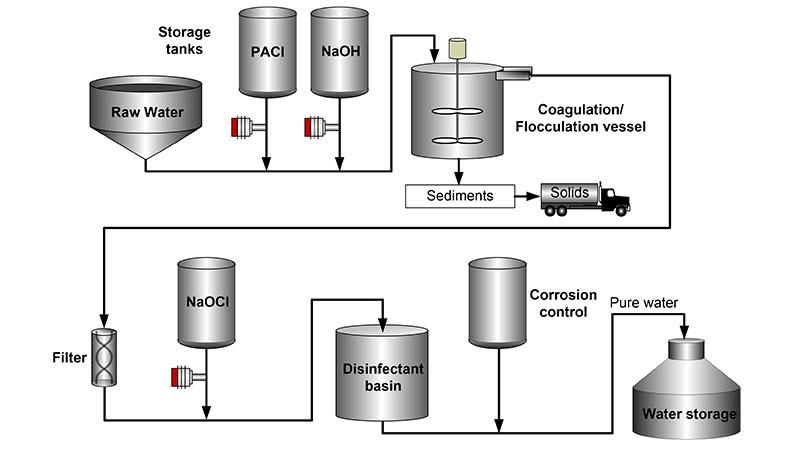
Water treatment by chemical precipitation is a complex process. It starts with adding flocculants, specifically, Polyaluminium Chloride (PACl) and Sodium Hydroxide (NaOH) into raw water. PACl is a synthetic polymer dissolved in water. It precipitates in big volumetric flocs which absorb suspended pollutants in the raw water. The amount of Polyaluminium Chloride to be added to the process is defined by the turbidity of the raw water. In order to keep the flocculation process smooth, PACl concentration must be higher than 10 %. Polyaluminium Chloride is stable in the storage tank, however, it tends to crystallize after a period of time. Vaisala Polaris™ Refractometer monitors the concentration of PACl to inform about the need for tank or pipe cleaning, thus preventing blockage caused by the PACl crystals.
NaOH regulates pH level, increases alkalinity and neutralizes acids in the water. In alkaline water the coagulation and flocculation processes work more effectively. Moreover, sufficient alkalinity prevents dissolving the lead from pipes and pipe fittings, as well as reducing the corrosiveness effect of the water to iron pipes.
Further, particles suspended in water start to precipitate and agglomerate to form larger particles, known as flocs. The flocs are then settled at the bottom forming a sludge. The sediment is removed from the process. After separating most of the floc, the water is filtered to remove the remaining suspended particles and unsettled floc.
In the filtration phase the water goes through the layers of anthracite, sand and gravel. Organic compounds contributing to taste and odor are removed. Other remaining particles are trapped by adhering to the sand and gravel particles.
After harmful micro-organisms have been filtered, it is necessary to add disinfecting chemicals to the water in order to inactivate any remaining pathogens and potentially harmful micro-organisms. One of the disinfecting chemicals used is Sodium Hypochlorite (NaOCl). This chemical releases chlorine when it is dissolved in water, which is an efficient and safe disinfectant if added in a sufficient amount. Apart from sodium hypochlorite, liquid chlorine and chlorine dioxide may also be used as disinfectants.
Fluoride may also be added to the water with the goal of reducing tooth decay and preventing chronic diseases. However, fluoride in the water must not exceed recommended levels. Excessive levels of fluoride can be toxic or cause undesirable cosmetic effects such as staining of teeth.
Sodium Hypochlorite is unstable and easily decomposes. The stability of NaOCl solution is dependent on the following factors: hypochlorite concentration, temperature of the solution, pH value of the solution, concentration of the impurities catalyzing decomposition, and exposure to light. With the refractometer it is possible to monitor NaOCl concentration and control the disinfection conditions.
The water purification disinfection stage is accomplished in the disinfectant basin. In order to assure high quality of the purified water corrosion control is performed. Finally, the pure water is stored for further consumption.
Instrumentation and installation
Vaisala Polaris™ PR53M Process Refractometer provides in-line measurements of Polyaluminium Chloride and Sodium Hydroxide at the initial stage of the purification process, ensuring the efficient flocculation of undesired particles. By measuring Sodium Hypochlorite and Fluoride at the water disinfection stage a high-quality purified water at the outlet is assured.
The refractometer is installed in three different points in a by-pass loop between each chemical storage tank pump outlet and the treatment point. The refractometer allows monitoring of the chemicals concentration at the exit from the storage tank to the pipe treatment point.
- Typical measurement range of PACl is ca. 10-11 %.
- Typical measurement range of NaOH is ca. 40-45 %.
- Typical measurement range of NaOCl is ca. 8-12 %.

Vaisala Polaris™ PR53M Process Refractometer
Description
The Vaisala Polaris PR53M PTFE-Body Process Refractometer is for chemically aggressive solutions and ultra-pure fine chemical processes. The PR53M can be mounted to ½ inch process lines with a standard NTP-threaded connection, or optional pillar or flare fittings.
Measurement range
Refractive Index (nD) 1.3200 – 1.5300, corresponding to 0-100 % by weight